Glycemic Management of Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Semaglutide Versus Liraglutide for Treatment of Obesity
Archives of Diabetes & Obesity DOI: 10.32474/ADO.2021.03.000162 ISSN: 2638-5910 Review Article Semaglutide versus liraglutide for treatment of obesity Nasser Mikhail* *Department of Medicine, Endocrinology Division, David-Geffen UCLA Medical School, USA *Corresponding author: Nasser Mikhail, Endocrinology Division, Department of Medicine, Olive View-UCLA Medical Center, David- Geffen UCLA Medical School, CA, USA Received: April 02, 2021 Published: April 19, 2021 Abstract Background: Once weekly (OW) semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) currently under evaluation for treatment of obesity at a dose of 2.4 mg OW. Objective Methods : To compare weight-loss efficacy and safety of once daily (OD) liraglutide 3.0 mg versus OW semaglutide 2.4 mg. : Pubmed research up to March 31, 2021. Randomized trials, pertinent animal studies, and reviews are included. Search Results terms were glucagon-like peptide-1 receptor agonists, weight loss, obesity, liraglutide, semaglutide, efficacy, safety. semaglutide 2.4 mg. However, marked resemblance between trials in terms of study protocols and subjects’ characteristics may allow indirect: No comparison. head to head In clinical trials are trials available of OW tosemaglutide, provide direct this comparison drug was consistently of efficacy ofassociated OD liraglutide with greater 3.0 mg weightversus lossOW than in trials of OD liraglutide. Thus, placebo-corrected percentage weight reduction was -10.3 to -12.4% and -5.4% with OW semaglutide and OD liraglutide, respectively. In patients with type 2 diabetes, corresponding weight reduction was less pronounced with both drugs being -6.2% and -4.3% with OW semaglutide and OD liraglutide, respectively. -

GLP-1 Receptor Agonists
Cognitive Vitality Reports® are reports written by neuroscientists at the Alzheimer’s Drug Discovery Foundation (ADDF). These scientific reports include analysis of drugs, drugs-in- development, drug targets, supplements, nutraceuticals, food/drink, non-pharmacologic interventions, and risk factors. Neuroscientists evaluate the potential benefit (or harm) for brain health, as well as for age-related health concerns that can affect brain health (e.g., cardiovascular diseases, cancers, diabetes/metabolic syndrome). In addition, these reports include evaluation of safety data, from clinical trials if available, and from preclinical models. GLP-1 Receptor Agonists Evidence Summary GLP-1 agonists are beneficial for patients with type 2 diabetes and obesity. Some evidence suggests benefits for Alzheimer’s disease. It is unclear whether it is beneficial for individuals without underlying metabolic disease. Semaglutide seems to be most effective for metabolic dysfunction, though liraglutide has more preclinical data for Alzheimer’s disease. Neuroprotective Benefit: Evidence from many preclinical studies and a pilot biomarker study suggest some neuroprotective benefits with GLP-1 agonists. However, whether they may be beneficial for everyone or only a subset of individuals (e.g. diabetics) is unclear. Aging and related health concerns: GLP-1 agonists are beneficial for treating diabetes and cardiovascular complications relating to diabetes. It is not clear whether they have beneficial effects in otherwise healthy individuals. Safety: GLP-1 agonists are generally safe for most people with minor side effects. However, long-term side effects are not known. 1 Availability: Available Dose: Varies - see Chemical formula: C172H265N43O51 (Liraglutide) as a prescription chart at the end of MW: 3751.262 g/mol medicine. -

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Treatment of Diabetes Mellitus
TREATMENT OF DIABETES MELLITUS DIABETES is a condition that affects how the body makes energy from food. Food is broken down into sugar (glucose) in the body and released into the blood. When the blood sugar level rises after a meal, insulin responds to let the sugar into the cells to be used as energy. In diabetes, the body either does not make enough insulin or it stops responding to insulin as well as it should. This results in sugar staying in the blood and leads to serious health problems over time. DIAGNOSIS OF DIABETES1 • A1C Test: Lab test measuring average blood sugar over past two to three months • Fasting Blood Sugar Test: Lab test measuring blood sugar after eight hours of no food or drink • Oral Glucose Tolerance Test (OGTT): Measures blood sugar before and two hours after drinking a specific sugary liquid • Random Blood Sugar Test: Measures blood sugar at a moment in time, without any kind of preparation (like fasting) FASTING BLOOD ORAL GLUCOSE TOLERANCE RANDOM BLOOD RESULT A1C TEST SUGAR TEST TEST SUGAR TEST Diabetes ≥ 6.5% ≥126 mg/dL ≥ 200 mg/dL ≥ 200 mg/dL Prediabetes 5.7 – 6.4% 100 – 125 mg/dL 140 – 199 mg/dL N/A Normal < 5.7% ≤99 mg/dL < 140 mg/dL N/A NON-DRUG TREATMENTS2 THERAPY COST WHAT TO EXPECT Diet (Mediterranean diet) and exercise (30 minutes a day, five days a week of moderate- Weight loss $-$$ intensity exercise); 7% weight loss decreases risk of diabetes3 Psychological intervention $$-$$$ Psychotherapy may reduce diabetic distress and improve glycemic control4,5 nationalcooperativerx.com PRESCRIPTION TREATMENTS -

295-304 Research Article Pulsatile Drug Delivery of Chitosan Co
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(12):295-304 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Pulsatile drug delivery of chitosan coated beads of miglitol with fast dissolving glimepiride tablet Bhise S. H.*, Surve B. S., Aloorkar N. H., Majumdar S. H. and Kulkarni A. S. Department of Pharmaceutics, Satara College of Pharmacy, Satara, Shivaji University, Maharashtra, India _____________________________________________________________________________________________ ABSTRACT : Pulsatile drug delivery system was developed which have three parts fast dissolving tablet of glimepiride, sustained release chitosan coated microbeads of miglitol and plug of HPMC E5 and spray dried lactose. After pre- formulation studies fast dissolving tablets were prepared by direct compression method; which shows instant drug release and % CDR of glimepiride fast dissolving tablet was found to be 70.81%. polymer plug have lag time 2.30 hr, chitosan coated miglitol beads shows sustained release upto 81.66% .this system is evaluated using different physicochemical parameters and in-vitro studies. Result suggests that the system can be applicable for diabetes treatment. Key words: Pulsatile, microbeads, sustained release, miglitol, glimepiride. _____________________________________________________________________________________________ INTRODUCTION Oral controlled drug delivery systems represent the most popular form of controlled drug delivery system which release the drug with constant or variable release rates.[1] Dose of drug, reduced dosage frequency, avoidance of side effects, and improved patient compliance. However, there are certain conditions for which such a release pattern is not suitable. These conditions demand release of drug after a lag time. In other words, it is required that the drug should not be released at all during the initial phase of dosage form administration. -

Treatment Patterns, Persistence and Adherence Rates in Patients with Type 2 Diabetes Mellitus in Japan: a Claims- Based Cohort Study
Open access Research BMJ Open: first published as 10.1136/bmjopen-2018-025806 on 1 March 2019. Downloaded from Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims- based cohort study Rimei Nishimura,1 Haruka Kato,2 Koichi Kisanuki,2 Akinori Oh,2 Shinzo Hiroi,2 Yoshie Onishi,3 Florent Guelfucci,4 Yukio Shimasaki2 To cite: Nishimura R, Kato H, ABSTRACT Strengths and limitations of this study Kisanuki K, et al. Treatment Objective To determine real-world trends in antidiabetic patterns, persistence and drug use, and persistence and adherence, in Japanese ► This retrospective evaluation of administrative adherence rates in patients patients with type 2 diabetes mellitus (T2DM). with type 2 diabetes mellitus claims data (2011–2015) using the Japan Medical Design Retrospective evaluation of administrative claims in Japan: a claims-based Data Center (JMDC) and Medical Data Vision (MDV) data (2011–2015) using the Japan Medical Data Center cohort study. BMJ Open databases was conducted to determine real-world (JMDC) and Medical Data Vision (MDV) databases. 2019;9:e025806. doi:10.1136/ trends in antidiabetic drug use, and persistence and Setting Analysis of two administrative claims databases bmjopen-2018-025806 adherence, in Japanese patients with type 2 dia- for Japanese patients with T2DM. betes mellitus (T2DM); 40 908 and 90 421 patients ► Prepublication history and Participants Adults (aged ≥18 years) with an International additional material for this were included from the JMDC and MDV databases, Classification of Diseases, 10th Revision code of T2DM and paper are available online. To respectively. at least one antidiabetic drug prescription. -

(Pram) and Insulin A21G Improves Post-Prandial Glucose Vs Novolog
ADO09, A Co-Formulation Of Pramlintide (Pram) and Insulin A21G improves Post-Prandial Glucose Vs Novolog® in Type 1 Diabetes (T1DM) G.Meiffren¹, G.Andersen², R.Eloy¹, C.Seroussi¹, C.Mégret¹, S.Famulla², Y.-P Chan¹, M.Gaudier¹, O.Soula¹, J.H. DeVries²,T.Heise² (1 Adocia, Lyon, France ; 2 Profil, Neuss, Germany) Introduction & Background Overall safety Outpatient period results - CGM metrics o ADO09 (M1Pram) is a co-formulation of pramlintide and insulin A21G o Both treatments were well tolerated without any treatment-related serious adverse events o Most of the CGM metrics (TiR [70-180], TiR [80-140], mean blood glucose per day), were significantly improved developed to leverage the beneficial effects of pramlintide on post-prandial (Table 2). As expected M1Pram had numerically more, mostly gastrointestinal adverse events with M1Pram (Table 4). Postprandial and mean 24-hour glucose profiles were improved with M1Pram (Fig. 3) glucose without additional injections than insulin aspart Table 4: CGM metrics, all days. Significant differences are marked in bold Objective and design o No severe hypoglycemia were seen, slightly more hypoglycemic events occurred with M1Pram Ratio of LSMean* o To compare the effect of M1Pram and insulin aspart (Novolog®, Novo than with aspart (Table 3) Difference Parameter Treatment LS Mean M1Pram / Aspart P-value Nordisk) on post-prandial glucose control, glycemic control assessed by Table 2: Incidence of adverse events throughout the trial (M1Pram-Aspart) (95% CI) CGM and safety/tolerability M1Pram Aspart M1Pram -

TRULICITY, INN-Dulaglutide
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Trulicity 0.75 mg solution for injection in pre-filled pen Trulicity 1.5 mg solution for injection in pre-filled pen Trulicity 3 mg solution for injection in pre-filled pen Trulicity 4.5 mg solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Trulicity 0.75 mg solution for injection in pre-filled pen Each pre-filled pen contains 0.75 mg of dulaglutide* in 0.5 ml solution. Trulicity 1.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 1.5 mg of dulaglutide* in 0.5 ml solution. Trulicity 3 mg solution for injection in pre-filled pen Each pre-filled pen contains 3 mg of dulaglutide* in 0.5 ml solution. Trulicity 4.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 4.5 mg of dulaglutide* in 0.5 ml solution. *produced in CHO cells by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Type 2 Diabetes Mellitus Trulicity is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. -

A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients with Type 2 Diabetes
1926 Diabetes Care Volume 41, September 2018 Ildiko Lingvay,1 Cyrus V. Desouza,2 A 26-Week Randomized Katarina S. Lalic,3 Ludger Rose,4 Thomas Hansen,5 Jeppe Zacho,5 and Controlled Trial of Semaglutide Thomas R. Pieber6 EMERGING THERAPIES: DRUGS AND REGIMENS Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin Diabetes Care 2018;41:1926–1937 | https://doi.org/10.2337/dc17-2381 OBJECTIVE To investigate the efficacy and safety of once-daily semaglutide in comparison with once-daily liraglutide and placebo in patients with type 2 diabetes. RESEARCH DESIGN AND METHODS This 26-week, multicenter, double-blind trial involved patients diagnosed with 1University of Texas Southwestern Medical Cen- type 2 diabetes with HbA1c 7.0–10.0% (53–86 mmol/mol) and treated with diet ter at Dallas, Dallas, TX and exercise with or without metformin. Patients were randomized 2:2:1 to once- 2University of Nebraska Medical Center, Omaha, daily semaglutide, liraglutide, or placebo in one of four volume-matched doses NE 3Faculty of Medicine, University of Belgrade, and (semaglutide 0.05, 0.1, 0.2, or 0.3 mg and liraglutide 0.3, 0.6, 1.2, or 1.8 mg, with Clinic for Endocrinology, Diabetes and Metabolic both compared within each volume-matched dose group). Primary end point was Diseases, Clinical Center of Serbia, Belgrade, Serbia change in HbA1c from baseline to week 26. 4Institute for Diabetes Research in Munster,¨ RESULTS Munster,¨ Germany 5Novo Nordisk A/S, Søborg, Denmark In total, 705 randomized patients were exposed to trial products. -

In-Vitro Anti-Diabetic Activity and In-Silico Studies of Binding Energies
Pharmacia 67(4): 363–371 DOI 10.3897/pharmacia.67.e58392 Research Article In-vitro anti-diabetic activity and in-silico studies of binding energies of palmatine with alpha-amylase, alpha-glucosidase and DPP-IV enzymes Patrick Okechukwu1, Mridula Sharma1, Wen Hui Tan1, Hor Kuan Chan1, Kavita Chirara1, Anand Gaurav1, Mayasah Al-Nema1 1 UCSI Universit, Kuala Lumpur, Malaysia Corresponding author: Patrick Okechukwu ([email protected]) Received 5 September 2020 ♦ Accepted 18 October 2020 ♦ Published 27 November 2020 Citation: Okechukwu P, Sharma M, Tan WH, Chan HK, Chirara K, Gaurav A, Al-Nema M (2020) In-vitro anti-diabetic activity and in-silico studies of binding energies of palmatine with alpha-amylase, alpha-glucosidase and DPP-IV enzymes. Pharmacia 67(4): 363–371. https://doi.org/10.3897/pharmacia.67.e58392 Abstract Palmatine a protoberberine alkaloid has been previously reported to possess in vivo antidiabetic and antioxidant property. The aim of the experiment is to evaluate the in vitro antidiabetic activity and in-silico studies of the binding energies of Palmatine, acarbose, and Sitagliptin with the three enzymes of alpha-amylase, alpha-glucosidase, and dipeptidyl peptidase-IV (DPP-IV). The in vitro antidiabetic study was done by evaluating the inhibitory effect of palmatine on the activities of alpha-amylase, alpha-glucosidase, and DPP-IV. Acarbose, and sitagliptin was used as standard drug. The molecular docking study was performed to study the binding interactions of palmatine with alpha-glucosidase, a-amylase, and DPP-IV. The binding interactions were compared with the standard compounds Sitagliptin and acarbose. Palmatine with IC50 (1.31 ± 0.27 µM) showed significant difference of (< 0.0001) higher inhib- iting effect on alpha-amylase and weak inhibiting effect on alpha-glucosidase enzyme with IC50 (9.39 ± 0.27 µM) and DPP-IV with IC50 (8.7 ± 1.82 µM). -
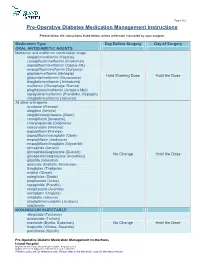
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic