Combination Use of Insulin and Incretins in Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Comparison of Adjunctive Therapy with Metformin and Acarbose in Patients with Type-1 Diabetes Mellitus
Original Article Comparison of adjunctive therapy with metformin and acarbose in patients with Type-1 diabetes mellitus Amir Ziaee1, Neda Esmailzadehha2, Maryam Honardoost3 ABSTRACT Objective: All the aforementioned data have stimulated interest in studying other potential therapies for T1DM including noninsulin pharmacological therapies. The present study attempts to investigate the effect of adjunctive therapy with metformin and acarbose in patients with Type-1 diabetes mellitus. Method: In a single-center, placebo-controlled study (IRCT201102165844N1) we compared the results of two clinical trials conducted in two different time periods on 40 patients with Type-1 diabetes mellitus. In the first section, metformin was given to the subjects.After six months, metformin was replaced with acarbose in the therapeutic regimen. In both studies, subjects were checked for their BMI, FBS, HbA1C, TGs, Cholesterol, LDL, HDL, 2hpp, unit of NPH and regular insulin variations. Results: Placebo-controlled evaluation of selected factors has showna significant decrease in FBS and TG levels in the metformin group during follow up but acarbose group has shown substantial influence on two hour post prandial (2hpp) and regular insulin intake decline.Moreover, Comparison differences after intervention between two test groups has shown that metformin has had superior impact on FBS and HbA1C decline in patients. Nonetheless, acarbose treatment had noteworthy influence on 2hpp, TGs, Cholesterol, LDL, and regular insulin intake control. Conclusion: The results of this experiment demonstrate that the addition of acarbose or metformin to patients with Type-1 diabetes mellitus who are controlled with insulin is commonly well tolerated and help to improve metabolic control in patients. -

Treatment of Diabetes Mellitus
TREATMENT OF DIABETES MELLITUS DIABETES is a condition that affects how the body makes energy from food. Food is broken down into sugar (glucose) in the body and released into the blood. When the blood sugar level rises after a meal, insulin responds to let the sugar into the cells to be used as energy. In diabetes, the body either does not make enough insulin or it stops responding to insulin as well as it should. This results in sugar staying in the blood and leads to serious health problems over time. DIAGNOSIS OF DIABETES1 • A1C Test: Lab test measuring average blood sugar over past two to three months • Fasting Blood Sugar Test: Lab test measuring blood sugar after eight hours of no food or drink • Oral Glucose Tolerance Test (OGTT): Measures blood sugar before and two hours after drinking a specific sugary liquid • Random Blood Sugar Test: Measures blood sugar at a moment in time, without any kind of preparation (like fasting) FASTING BLOOD ORAL GLUCOSE TOLERANCE RANDOM BLOOD RESULT A1C TEST SUGAR TEST TEST SUGAR TEST Diabetes ≥ 6.5% ≥126 mg/dL ≥ 200 mg/dL ≥ 200 mg/dL Prediabetes 5.7 – 6.4% 100 – 125 mg/dL 140 – 199 mg/dL N/A Normal < 5.7% ≤99 mg/dL < 140 mg/dL N/A NON-DRUG TREATMENTS2 THERAPY COST WHAT TO EXPECT Diet (Mediterranean diet) and exercise (30 minutes a day, five days a week of moderate- Weight loss $-$$ intensity exercise); 7% weight loss decreases risk of diabetes3 Psychological intervention $$-$$$ Psychotherapy may reduce diabetic distress and improve glycemic control4,5 nationalcooperativerx.com PRESCRIPTION TREATMENTS -

Does Addition of Vildagliptin to Metformin Monotherapy Improve Glycemic Control in Patients with Type 2 Diabetes Mellitus?
PRACTICE pOINT www.nature.com/clinicalpractice/endmet Does addition of vildagliptin to metformin monotherapy improve glycemic control in patients with type 2 diabetes mellitus? Original article Bosi E et al. (2007) Effects of vildagliptin on OUTCOME MEASURES glucose control over 24 weeks in patients with type 2 diabetes The primary outcome measure was the change inadequately controlled with metformin. Diabetes Care 30: 890–895 in HbA1c level from baseline to study end. Secondary outcome measures included FPG SYNOPSIS levels, lipid profiles, β-cell function, and the KEYWORDS dipeptidyl peptidase 4 inhibitor, incidence and severity of adverse events. glycemic control, HbA1c, type 2 diabetes mellitus, vildagliptin RESULTS BACKGROUND The 50 mg vildagliptin, 100 mg vildagliptin Metformin is frequently prescribed as the and placebo groups comprised 177, 185, and first-line treatment for type 2 diabetes mellitus 182 patients, respectively. Participants were (T2DM); however, additional antidiabetic agents predominantly white and obese, with a mean might be required when glycemic control is age of 54 years. The mean duration of T2DM poor. was 6.2 years, the mean duration of metformin monotherapy was 17 months, and the mean OBJECTIVE metformin dose was 2,100 mg daily. The study To assess the efficacy and safety of the dipeptidyl was completed by >83% of patients in each peptidase 4 (DPP4) inhibitor vildagliptin as group. The mean baseline HbA1c level was 8.4% an add-on to metformin monotherapy. and the mean baseline FPG level was 9.9 mmol/l. Treatment with vildagliptin improved both of DESIGN AND INTERVENTION these parameters. The between-treatment This was a 24-week, multicenter, double- difference (vildagliptin minus placebo) in HbA1c blind, placebo-controlled, randomized study at study end was –0.7% and –1.1% with 50 mg of patients with T2DM inadequately controlled and 100 mg vildagliptin, respectively (P <0.001 with metformin monotherapy. -

Galvus Data Sheet
1 NEW ZEALAND DATA SHEET 1 GALVUS (50 mg tablets) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Vildagliptin: 1-[(3-Hydroxy-adamant-1-ylamino)-acetyl]-pyrrolidine-2(S)-carbonitrile. One tablet of Galvus® contains 50 mg of vildagliptin. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Galvus 50 mg: white to light yellowish, round (8 mm diameter) flat faced with beveled edges, unscored tablet. One side is debossed with "NVR", and the other side with "FB". 4 CLINICAL PARTICULARS 4.1 Therapeutic indications Galvus is indicated as an adjunct to diet and exercise to improve glycaemic control in patients with type 2 diabetes mellitus. • as monotherapy. • in dual combination with metformin, a sulphonylurea (SU), or a thiazolidinedione (TZD) when diet, exercise and a single antidiabetic agent do not result in adequate glycaemic control. • In triple combination with a sulphonylurea and metformin when diet and exercise plus dual therapy with these agents do not provide adequate glycaemic control. • In combination with insulin (with or without metformin) when diet, exercise and a stable dose of insulin do not result in adequate glycaemic control. 4.2 Dosage and method of administration Dose The management of antidiabetic therapy should be individualized. The recommended dose of Galvus is 50 mg once or twice daily. The maximum daily dose of Galvus is 100 mg. For monotherapy, and for combination with metformin, with a TZD or with insulin (with or without metformin), the recommended dose of Galvus is 50 mg or 100 mg daily. When used in dual combination with a sulphonylurea, the recommended dose of vildagliptin is 50 mg once daily. -

Supplementary Material
Supplementary material Table S1. Search strategy performed on the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL). 1. Randomi*ed study OR random allocation OR Randomi*ed controlled trial OR Random* Control* trial OR RCT Epidemiological study 2. sodium glucose cotransporter 2 OR sodium glucose cotransporter 2 inhibitor* OR sglt2 inhibitor* OR empagliflozin OR dapagliflozin OR canagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR sotagliflozin OR sergliflozin OR remogliflozin 3. 1 AND 2 1 Table S2. Safety outcomes of empagliflozin and linagliptin combination therapy compared with empagliflozin or linagliptin monotherapy in treatment naïve type 2 diabetes patients Safety outcome Comparator 1 Comparator 2 I2 RR [95% CI] Number of events Number of events / / total subjects total subjects i. Empagliflozin + linagliptin vs empagliflozin monotherapy Empagliflozin + Empagliflozin linagliptin monotherapy ≥ 1 AE(s) 202/272 203/270 77% 0.99 [0.81, 1.21] ≥ 1 drug-related 37/272 38/270 0% 0.97 [0.64, 1.47] AE(s) ≥ 1 serious AE(s) 13/272 19/270 0% 0.68 [0.34, 1.35] Hypoglycaemia* 0/272 5/270 0% 0.18 [0.02, 1.56] UTI 32/272 25/270 29% 1.28 [0.70, 2.35] Events suggestive 12/272 13/270 9% 0.92 [0.40, 2.09] of genital infection i. Empagliflozin + linagliptin vs linagliptin monotherapy Empagliflozin + Linagliptin linagliptin monotherapy ≥ 1 AE(s) 202/272 97/135 0% 1.03 [0.91, 1.17] ≥ 1 drug-related 37/272 17/135 0% 1.08 [0.63, 1.84] AE(s) ≥ 1 serious AE(s) 13/272 2/135 0% 3.22 [0.74, 14.07] Hypoglycaemia* 0/272 1/135 NA 0.17 [0.01, 4.07] UTI 32/272 12/135 0% 1.32 [0.70, 2.49] Events suggestive 12/272 4/135 0% 1.45 [0.47, 4.47] of genital infection RR, relative risk; AE, adverse event; UTI, urinary tract infection. -

Januvia (Sitagliptin) Tablets
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: NDA 021995/S-013 Trade Name: JANUVIA Generic Name: Sitagliptin Sponsor: Merck & Co., Inc. Approval Date: 12/28/2009 Indications: JANUVIA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: NDA 021995/S-013 CONTENTS Reviews / Information Included in this NDA Review. Approval Letter X Other Action Letters X Labeling X Summary Review Officer/Employee List Office Director Memo Cross Discipline Team Leader Review Medical Review(s) X Chemistry Review(s) Environmental Assessment Pharmacology Review(s) X Statistical Review(s) Microbiology Review(s) Clinical Pharmacology/Biopharmaceutics Review(s) Risk Assessment and Risk Mitigation Review(s) X Proprietary Name Review(s) Other Review(s) X Administrative/Correspondence Document(s) X CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: NDA 021995/S-013 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021995/S-013 SUPPLEMENT APPROVAL Merck & Co., Inc. Attention: Richard J. Swanson, Ph.D. Director, Regulatory Affairs P.O. Box 1000, UG2C-50 North Wales, PA 19454-1099 Dear Dr. Swanson: Please refer to your supplemental new drug application (S-013) dated and received March 5, 2009, submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FDCA) for Januvia (sitagliptin) tablets. We also refer to your supplemental new drug application (b) (4) dated and received November 13, 2009. Your submission of November 13, 2009, also constitutes a complete response to our October 16, 2009, action letter for supplemental application S-013. -

Pharmacokinetics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 Inhibitor
Available online a t www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2016, 8(12):292-295 CODEN (USA): PCHHAX (http://derpharmachemica.com/archive.html) Mini-review: Pharmacokinetics of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor Nermeen Ashoush a,b aClinical Pharmacy and Pharmacy Practice Department, Faculty of Pharmacy, British University in Egypt, El- Sherouk city, Cairo 11837, Egypt. bHead of Health Economics Unit, Center for Drug Research and Development (CDRD), Faculty of Pharmacy, British University in Egypt, El-Sherouk city, Cairo 11837, Egypt. _____________________________________________________________________________________________ ABSTRACT The dipeptidyl peptidase-4 (DPP-4) inhibitors are novel oral hypoglycemic drugs which have been in clinical use for the past 10 years. The drugs are safe, weight neutral and widely prescribed. There are currently many gliptins approved by FDA, namely sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin with several more in advanced stages of development. The gliptins may possess cardiovascular protective effects and their administration may promote β-cell survival; claims currently being evaluated in clinical and preclinical studies. The gliptins are an optional second-line therapy after metformin; they are generally well tolerated with low risk of hypoglycemia. The various compounds differ with respect to their pharmacokinetic properties; however, their clinical efficacy appears to be similar. The clinical differences between the various compounds -

Vildagliptin/Metformin Hydrochloride Novartis, INN-Vildagliptin/Metformin
European Medicines Agency Evaluation of Medicines for Human Use Doc.Ref.: EMEA/CHMP/656453/2008 ASSESSMENT REPORT FOR Vildagliptin/metformin hydrochloride Novartis International Nonproprietary Name: vildagliptin / metformin hydrochloride Procedure No. EMEA/H/C/001050 Assessment Report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 84 16 E-mail: [email protected] http://www.emea.europa.eu © European Medicines Agency, 2008. Reproduction is authorised provided the source is acknowledged TABLE OF CONTENTS 1. BACKGROUND INFORMATION ON THE PROCEDURE........................................... 3 1.1 Submission of the dossier ........................................................................................................ 3 1.2 Steps taken for the assessment of the product.......................................................................... 3 2. SCIENTIFIC DISCUSSION................................................................................................. 4 2.1 Introduction.............................................................................................................................. 4 2.2 Quality aspects......................................................................................................................... 4 2.3 Non-clinical aspects................................................................................................................. 4 2.4 Clinical -
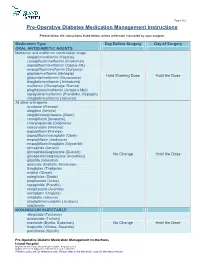
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

Albiglutide Needs Greater Accuracy from Remaining Shots on Goal
November 18, 2011 Albiglutide needs greater accuracy from remaining shots on goal Evaluate Vantage Victoza is looking increasingly bullet-proof. Novo Nordisk’s once-daily GLP-1 agonist proved superior to GlaxoSmithKline’s once-weekly version, albiglutide, in reducing blood glucose while also showing significant weight loss advantages in type II diabetics. This follows a similar win for Victoza against Amylin Pharmaceuticals’ once-weekly challenger in Bydureon (Amylin scores own goal in Bydureon vs Victoza head-to- head study, March 3, 2011). Albiglutide still has a number of shots on goal – data this week from the Harmony 7 trial was the first from eight phase III studies all nearing completion – with a head-to-head against Merck & Co’s DPP-IV inhibitor Januvia probably the most significant in terms of determining albiglutide’s future role and potential in the diabetes market. Although expectations have never been that high, Glaxo’s product had an opportunity to capitalise on the setbacks to Byetta, Bydureon and Roche’s once-weekly taspoglutide – this first trial read out suggests that opportunity will be limited at best or missed altogether. Playing catch up Glaxo’s decision almost three years ago to embark on an extensive phase III programme for albiglutide, branded Syncria at the time, was seen as a bit of a gamble (Glaxo makes brave decision with phase III trials for Syncria, February 17, 2009). Safety concerns about Amylin’s twice-daily Byetta, the only approved GLP-1 agonist at the time, and the fact albiglutide had a lot of ground to make up on Bydureon and taspoglutide, suggested an uphill struggle for Glaxo’s product which was licensed from Human Genome Sciences in 2004. -

Soliqua – Criteria
Criteria Based Consultation Prescribing Program CRITERIA FOR DRUG COVERAGE Insulin glargine; lixisenatide (Soliqua) subcutaneous injection Initiation (new start) criteria: Non-formulary lixisenatide/glargine (Soliqua) will be covered on the prescription drug benefit when the following criteria are met: Must meet criteria for both individual agents lixisenatide and insulin glargine. Criteria for lixisenatide: • Diagnosis of Type 2 Diabetes Mellitus • Intolerance to preferred GLP-1 agonists liraglutide (Victoza) AND injectable semaglutide (Ozempic) • No personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) • No personal history of gastroparesis • On maximally tolerated metformin dose (dose appropriate per renal function) or allergy or intolerance* to metformin (includes both metformin IR and XR) • And meets one of the following criteria: o Inadequate glycemic response on both basal and bolus insulin despite high dose requirements (total daily insulin dose of 1.5 units per kilogram of body weight or more OR greater than 200 units) OR o Has experienced recurrent nocturnal hypoglycemia with basal insulin defined as: 3 or more episodes of nocturnal hypoglycemia (BG less than 70 mg/dL) over the preceding 30 days that persists despite basal insulin dose reduction (including decrease in NPH dose and subsequent switch to and dose adjustment of insulin glargine) Criteria for insulin glargine • Use in patients with type 1 diabetes mellitus as basal insulin -OR- • Use in patients with