Or Repaglinide-Based Combination Therapy with Alogliptin in Drug-Naïve Patients with Type 2 Di
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment of Diabetes Mellitus
TREATMENT OF DIABETES MELLITUS DIABETES is a condition that affects how the body makes energy from food. Food is broken down into sugar (glucose) in the body and released into the blood. When the blood sugar level rises after a meal, insulin responds to let the sugar into the cells to be used as energy. In diabetes, the body either does not make enough insulin or it stops responding to insulin as well as it should. This results in sugar staying in the blood and leads to serious health problems over time. DIAGNOSIS OF DIABETES1 • A1C Test: Lab test measuring average blood sugar over past two to three months • Fasting Blood Sugar Test: Lab test measuring blood sugar after eight hours of no food or drink • Oral Glucose Tolerance Test (OGTT): Measures blood sugar before and two hours after drinking a specific sugary liquid • Random Blood Sugar Test: Measures blood sugar at a moment in time, without any kind of preparation (like fasting) FASTING BLOOD ORAL GLUCOSE TOLERANCE RANDOM BLOOD RESULT A1C TEST SUGAR TEST TEST SUGAR TEST Diabetes ≥ 6.5% ≥126 mg/dL ≥ 200 mg/dL ≥ 200 mg/dL Prediabetes 5.7 – 6.4% 100 – 125 mg/dL 140 – 199 mg/dL N/A Normal < 5.7% ≤99 mg/dL < 140 mg/dL N/A NON-DRUG TREATMENTS2 THERAPY COST WHAT TO EXPECT Diet (Mediterranean diet) and exercise (30 minutes a day, five days a week of moderate- Weight loss $-$$ intensity exercise); 7% weight loss decreases risk of diabetes3 Psychological intervention $$-$$$ Psychotherapy may reduce diabetic distress and improve glycemic control4,5 nationalcooperativerx.com PRESCRIPTION TREATMENTS -

295-304 Research Article Pulsatile Drug Delivery of Chitosan Co
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(12):295-304 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Pulsatile drug delivery of chitosan coated beads of miglitol with fast dissolving glimepiride tablet Bhise S. H.*, Surve B. S., Aloorkar N. H., Majumdar S. H. and Kulkarni A. S. Department of Pharmaceutics, Satara College of Pharmacy, Satara, Shivaji University, Maharashtra, India _____________________________________________________________________________________________ ABSTRACT : Pulsatile drug delivery system was developed which have three parts fast dissolving tablet of glimepiride, sustained release chitosan coated microbeads of miglitol and plug of HPMC E5 and spray dried lactose. After pre- formulation studies fast dissolving tablets were prepared by direct compression method; which shows instant drug release and % CDR of glimepiride fast dissolving tablet was found to be 70.81%. polymer plug have lag time 2.30 hr, chitosan coated miglitol beads shows sustained release upto 81.66% .this system is evaluated using different physicochemical parameters and in-vitro studies. Result suggests that the system can be applicable for diabetes treatment. Key words: Pulsatile, microbeads, sustained release, miglitol, glimepiride. _____________________________________________________________________________________________ INTRODUCTION Oral controlled drug delivery systems represent the most popular form of controlled drug delivery system which release the drug with constant or variable release rates.[1] Dose of drug, reduced dosage frequency, avoidance of side effects, and improved patient compliance. However, there are certain conditions for which such a release pattern is not suitable. These conditions demand release of drug after a lag time. In other words, it is required that the drug should not be released at all during the initial phase of dosage form administration. -

Effect of the Α-Glucosidase Inhibitor Miglitol on the Glucose Profile in Japanese Type 2 Diabetic Patients Receiving Multiple Daily Insulin Injections
Endocrine Journal 2012, 59 (4), 345-352 ORIGINAL Effect of the α-glucosidase inhibitor miglitol on the glucose profile in Japanese type 2 diabetic patients receiving multiple daily insulin injections Hiroyuki Kato, Akio Ohta, Suzuko Kobayashi, Satoshi Ishii, Yukiyoshi Sada, Hidetoshi Kobayashi, Shintaro Ohmori, Akihiko Kondo, Takuyuki Katabami, Junro Fuse, Hisashi Fukuda, Yoshio Nagai and Yasushi Tanaka Department of Internal Medicine, Division of Metabolism and Endocrinology, St. Marianna University School of Medicine, Kawasaki 216-8511, Japan Abstract. Strict postprandial glycemic control may have a preventive effect on atherogenesis in patients with type 2 diabetes. The α-glucosidase inhibitor (α-GI) miglitol is useful for controlling the early postprandial increase of glucose, but the combined effect of miglitol and multiple daily insulin injections (MDI) on glucose excursion has not been evaluated. First, we retrospectively compared the daily glucose profile, evaluated by self-monitoring of blood glucose (SMBG) at nine times on the day before discharge from hospital, between type 2 diabetic patients receiving MDI (n=81) or MDI plus miglitol at 150 mg daily (n=24). Second, we prospectively examined the effect of adding miglitol to MDI on the daily glucose profile (SMBG) in 19 other type 2 diabetic patients. Although the daily insulin dosage and the glucose level before meals did not differ between the two groups, the 1-h postprandial glucose level after each meal, 2-h glucose level after lunch and dinner, mean and standard deviation of glucose, and amplitude of glucose excursion were significantly lower or smaller in the MDI plus miglitol group than in the MDI group. -

Hypoglycemia in Diabetes: Common, Often Unrecognized
REVIEW ILAN GABRIELY, MD HARRY SHAMOON, MD CME Diabetes Research Center, Albert Einstein Professor of Medicine, Diabetes Research CREDIT College of Medicine, New York Center, Albert Einstein College of Medicine, New York Hypoglycemia in diabetes: Common, often unrecognized ■ ABSTRACT YPOGLYCEMIA poses a major barrier to H diabetes treatment. On one hand, we Hypoglycemic episodes in patients with diabetes often go want to maintain tight glycemic control to unrecognized, and over time, patients may lose the ability prevent the vascular complications of diabetes, to sense hypoglycemia, increasing their risk. Intensive but we also have to ensure the safety and com- diabetes control is beneficial for patients with diabetes, fort of the patient by avoiding hypoglycemia— but it increases their risk of hypoglycemia, underscoring and by recognizing and treating it if it occurs. the complexity of diabetes management. Hypoglycemic events are probably com- mon, especially in patients with type 1 diabetes. ■ KEY POINTS And when patients with type 2 diabetes receive insulin, they may become more prone to hypo- Epinephrine release during hypoglycemia becomes glycemic episodes. Unfortunately, the more progressively defective in type 1 diabetes. This decrease in episodes of hypoglycemia a patient has, the epinephrine response is accompanied by an attenuated more the body’s response is blunted, decreasing autonomic neural response, which results in the clinical the patient’s awareness of an episode. syndrome of impaired awareness of hypoglycemia (ie, Thus, we need to be vigilant in monitor- lack of the warning symptoms of prevailing ing patients for increasing episodes of hypo- glycemia, and for events that a patient may not hypoglycemia). -

Women with Higher BMI at Lower Risk for Glaucoma
44 WOMEN'S HEALTH SEPTEMBER 1, 2010 • FAMILY PRACTICE NEWS DRUGS, PREGNANCY, AND LACTATION Use of Antidiabetic Agents During Pregnancy n a previous column, I looked at the abinese), tolazamide (Tolinase), and tolbu- thetic analogue of human amylin given was discovered. No developmental toxi- ways uncontrolled hyperglycemia tamide (Orinase). They can cause marked by subcutaneous injection, but the ani- city was observed in the newborns. Iduring pregnancy causes significant and persistent neonatal hypoglycemia if mal data suggest moderate risk (struc- Pioglitazone (Actos) and rosiglitazone toxicity for the mother, embryo, fetus, taken close to birth. To prevent this toxi- tural anomalies in rats). The drug— (Avandia), thiazolidinediones used as ad- newborn, and adolescent (“Toxicity of city, therapy should be changed to insulin which slows the rate of gastric emptying, juncts to diet and exercise, lower insulin Diabetes in Pregnancy,” December in the third trimester or, at least, several prevents a postprandial rise in plasma resistance but do not promote insulin re- 2009, p. 52). days before birth. glucagon, and promotes satiety—is best lease. Animal reproduction data suggest This column examines the The second-generation sul- avoided in pregnancy. risk, but the human experience is too use of antidiabetic agents, fonylureas glipizide (Glu- Saxagliptin (Onglyza) and sitagliptin limited to assess the risk, so they should other than insulin, that are cotrol), glimepiride (Amaryl), (Januvia), inhibitors of the enzyme be avoided in pregnancy. used for non–insulin-depen- and glyburide (DiaBeta, Gly- dipeptidyl peptidase–4, are indicated as In summary, only metformin and gly- dent diabetes during preg- nase, and Micronase) are pre- monotherapy or in combination with buride have sufficient human pregnancy nancy and lactation. -
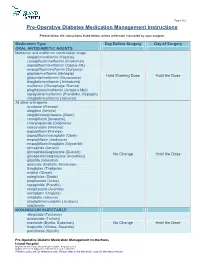
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

Comparative Review of Oral Hypoglycemic Agents in Adults
SECTION 18.5 Comparative Review of Oral Hypoglycemic Agents in Adults Harinder Chahal For WHO Secretariat Table of Contents Acronyms: ............................................................................................................................................................................... 3 I. Background and Rationale for the review: ....................................................................................................................... 4 II. Medications under comparative review: ......................................................................................................................... 4 Table 1 - New oral hypoglycemic agents for comparison with current EML agents .......................................................... 5 III. Literature searches and methodology: ............................................................................................................................ 5 1. Title Search Results: .................................................................................................................................................... 6 2. Statement about quality of evidence: ........................................................................................................................ 6 IV. Clinical efficacy and safety evaluation: ............................................................................................................................ 6 1. DPP-4 Inhibitors (Sitagliptin, Saxagliptin) and Metformin: ........................................................................................ -

NESINA (Alogliptin) Tablets, for Oral Use • Heart Failure: Consider the Risks and Benefits of NESINA Prior to Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use • Acute pancreatitis: There have been postmarketing reports of NESINA safely and effectively. See full prescribing information for acute pancreatitis. If pancreatitis is suspected, promptly NESINA. discontinue NESINA. (5.1) NESINA (alogliptin) tablets, for oral use • Heart failure: Consider the risks and benefits of NESINA prior to Initial U.S. Approval: 2013 initiating treatment in patients at risk for heart failure. If heart failure develops, evaluate and manage according to current ---------------------------RECENT MAJOR CHANGES-------------------------- standards of care and consider discontinuation of NESINA (5.2). Indications and Usage (1.1) 4/2016 • Hypersensitivity: There have been postmarketing reports of Dosage and Administration serious hypersensitivity reactions in patients treated with NESINA Patients with Renal Impairment (2.2) 4/2016 such as anaphylaxis, angioedema and severe cutaneous adverse Warnings and Precautions reactions, including Stevens-Johnson syndrome. In such cases, Pancreatitis (5.1) 4/2016 promptly discontinue NESINA, assess for other potential causes, Heart Failure (5.2) 4/2016 institute appropriate monitoring and treatment and initiate Hepatic Effects (5.4) 4/2016 alternative treatment for diabetes. (5.3) Bullous Pemphigoid (5.7) 12/2016 • Hepatic effects: Postmarketing reports of hepatic failure, ----------------------------INDICATIONS AND USAGE--------------------------- sometimes fatal. Causality cannot be excluded. If liver injury is NESINA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an detected, promptly interrupt NESINA and assess patient for adjunct to diet and exercise to improve glycemic control in adults with probable cause, then treat cause if possible, to resolution or type 2 diabetes mellitus. -

Oral Health Fact Sheet for Dental Professionals Adults with Type 2 Diabetes
Oral Health Fact Sheet for Dental Professionals Adults with Type 2 Diabetes Type 2 Diabetes ranges from predominantly insulin resistant with relative insulin deficiency to predominantly an insulin secretory defect with insulin resistance, American Diabetes Association, 2010. (ICD 9 code 250.0) Prevalence • 23.6 million Americans have diabetes – 7.8% of U.S. population. Of these, 5.7 million do not know they have the disease. • 1.6 million people ≥20 years of age are diagnosed with diabetes annually. • 90–95% of diabetic patients have Type 2 Diabetes. Manifestations Clinical of untreated diabetes • High blood glucose level • Excessive thirst • Frequent urination • Weight loss • Fatigue Oral • Increased risk of dental caries due to salivary hypofunction • Accelerated tooth eruption with increasing age • Gingivitis with high risk of periodontal disease (poor control increases risk) • Salivary gland dysfunction leading to xerostomia • Impaired or delayed wound healing • Taste dysfunction • Oral candidiasis • Higher incidence of lichen planus Other Potential Disorders/Concerns • Ketoacidosis, kidney failure, gastroparesis, diabetic neuropathy and retinopathy • Poor circulation, increased occurrence of infections, and coronary heart disease Management Medication The list of medications below are intended to serve only as a guide to facilitate the dental professional’s understanding of medications that can be used for Type 2 Diabetes. Medical protocols can vary for individuals with Type 2 Diabetes from few to multiple medications. ACTION TYPE BRAND NAME/GENERIC SIDE EFFECTS Enhance insulin Sulfonylureas Glipizide (Glucotrol) Angioedema secretion Glyburide (DiaBeta, Fluconazoles may increase the Glynase, Micronase) hypoglycemic effect of glipizide Glimepiride (Amaryl) and glyburide. Tolazamide (Tolinase, Corticosteroids may produce Diabinese, Orinase) hyperglycemia. Floxin and other fluoroquinolones may increase the hypoglycemic effect of sulfonylureas. -

Diabetes Recommendations and Tier Coverage Chart
DIABETES RECOMMENDATIONS AND TIER COVERAGE CHART The American Diabetes Association guidelines for 2020, recommend metformin as the preferred initial treatment for type 2 diabetes (T2DM) along with weight management and physical activity. In patients who have established ASVD or at high risk, CKD, or HF, a SGLT2i or GLP-1 receptor with proven efficacy is recommended independent of A1C. • ASCVD dominates: o GLP-1RA with proven CVD benefit (dulaglutide, liraglutide, injectable semaglutide) OR o SGLT2i with proven CVD benefit (canagliflozin, empagliflozin) if adequate eGFR • HF or CKD dominates: o SGLT2i with evidence of reducing HF and/or CKD progression (empagliflozin, canagliflozin, dapagliflozin) if adequate eGFR OR o If SGLT2i intolerant/contraindicated or eGFR is inadequate, then GLP-1RA with proven CVD benefit In individuals without established cardiovascular disease, pharmacological treatment should be patient-centered taking into account side-effects, cost, impact on weight, risk of hypoglycemia, and other patient preferences. For more detailed information regarding ADA recommendations for pharmacological agents to treat T2DM click here. The following chart is a list of oral and injectable diabetes medications listed by class with their respective A1C reduction and insurance coverage and/or coverage requirements for BCBS, HPHC, Tufts, TMP, and MassHealth. Tufts Medicare Medications BCBSMA HPHC Tufts Preferred MassHealth Biguanides A1C reduction: 1-1.5% metformin Tier 1 Tier 1;2 Tier 1 Tier 1 Covered Glucoghage (metformin) NC NC NC;Tier -

Comparison of Glimepiride, Alogliptin and Alogliptin+Pioglitazone Combination in Poorly Controlled Type 2 Diabetic Patients ( Protocol: Takeda ALO-IIT)
Takeda_ALO-IIT_Ver 3.3 date: 29/Dec/2016 Comparison of glimepiride, alogliptin and alogliptin+pioglitazone combination in poorly controlled type 2 diabetic patients ( Protocol: Takeda_ALO-IIT) Version No: 3.3 date:29/Dec/2016 Principal Investigator’s Affiliation: Seoul National University Bundang Hospital 1 Takeda_ALO-IIT_Ver 3.3 date: 29/Dec/2016 Principal Investigator’s Name: Sung Hee Choi Research Outline Comparison of glimepiride, alogliptin and alogliptin+pioglitazone combination in Title of Research poorly controlled type 2 diabetic patients Principal Investigator Professor Sung Hee Choi Institution Supporting Takeda Pharmaceuticals Korea Co. Ltd. Research Expenses The primary objective is to compare the change in HbA1c in week 24 in 3 treatment groups: the glimepiride monotherapy treatment group; the alogliptin monotherapy treatment group; the alogliptin - pioglitazone combination therapy treatment group. - The secondary objective is to compare the change in HbA1c in week 12 and fasting plasma glucose (FPG) in week 12 and 24 in the following 3 treatment groups over the course of 3 months (at the Baseline, in Week 12): (the glimepiride Research Objective monotherapy treatment group, the alogliptin monotherapy treatment group, and the alogliptin - pioglitazone combination therapy treatment group). - Also, the change in parameters of glycemic variability assessed by CGM will be investigated. - Also, for a 6-month period, the average change in the lipid profile will be compared (Baseline, Week 12, Week 24). · This trial is a three-armed, open label, random assignment trial. · The research subjects are patients who are first starting their treatment or patients who have failed with the metformin treatment and are changing their medication.