Diabetes: Putting It All Together to Design a High Value Diabetes Regimen for Your Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidenz Und Versorgungsrealität Von Kurzwirksamen Insulinanaloga in Der Behandlung Des Typ-2-Diabetes Mellitus
Evidenz und Versorgungsrealität von kurzwirksamen Insulinanaloga in der Behandlung des Typ-2-Diabetes mellitus – Eine Versorgungsanalyse auf der Basis von Sekundärdaten – Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften vorgelegt beim Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität in Frankfurt am Main von Matthias S. Pfannkuche aus Brühl Frankfurt am Main, im Jahr 2009 vom Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität als Dissertation angenommen. Dekan: Prof. Dr. rer. nat. Dieter Steinhilber Gutachter: Prof. Dr. rer. nat. Theo Dingermann Prof. Dr. rer. nat. Gerd Glaeske (Universität Bremen) Datum der Disputation: 15. Februar 2010 Bildnachweis Titelseite: Insulin Hexamer: http://commons.wikimedia.org/wiki/File:Human-insulin-hexamer-3D-ribbons.png (letzter Zugriff: 11.06.2009) Non semper ea sunt, quae videntur. (Phädrus, fabulae 4, 2, 5) Danksagung Diese Dissertation sowie die hiermit in Verbindung stehenden Publikationen wären ohne die Anregungen und Unterstützung durch viele Kollegen, Freunde und Organisationen nicht möglich gewesen. Ihnen möchte ich an dieser Stelle danken. Mein besonderer Dank gilt Prof. Dr. rer. nat Gerd Glaeske und Prof. Dr. rer. nat. Theo Dingermann, die diese Arbeit in vielerlei Hinsicht erst ermöglichten. Überaus dankbar bin ich Prof. Dr. rer. nat. Gerd Glaeske für die freundliche Aufnahme in seine Arbeitsgruppe, die es mir ermöglichte weitere Einblicke in die Gesundheitsökonomie, Gesundheitspolitik und Versorgungsforschung zu nehmen. Für das Korrekturlesen der kompletten Arbeit, die zahlreichen Hinweise und konstruktiven Diskussionen sowie die zahlreichen Mittagspausen danke ich im besonderen Dr. P.H. Falk Hoffmann. Herzlicher Dank gilt auch dem gesamten Arbeitskreis in Bremen sowie den Projektbeteiligten Krankenkassen, allen voran der GEK, die mir durch den Zugriff auf ihre Daten erst viele Analysen ermöglichten. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba
Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba by Kim¡ T. G. Guilbert A Thesis submitted to The Faculty of Graduate Studies in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Faculty of Pharmacy The University of Manitoba Winnipeg, Manitoba @ Kimi T.G. Guilbert, March 2005 TIIE UMYERSITY OF MANITOBA F'ACULTY OF GRADUATE STTJDIES +g+ù+ COPYRIGIIT PERMISSION PAGE Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba BY Kimi T.G. Guilbert A ThesisÆracticum submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfillment of the requirements of the degree of MASTER OF SCIENCE KIMI T.G. GTIILBERT O2()O5 Permission has been granted to the Library of The University of Manitoba to lend or sell copies of this thesis/practicum, to the National Library of Canada to microfïlm this thesis and to lend or sell copies of the film, and to University Microfilm Inc. to publish an abstract of this thesis/practicum. The author reserves other publication rights, and neither this thesis/practicum nor extensive extracts from it may be printed or otherwise reproduced without the author's written permission. Acknowledgements Upon initiation of this project I had a clear objective in mind--to learn more. As with many endeavors in life that are worthwhile, the path I have followed has brought me many places I did not anticipate at the beginning of my journey. ln reaching the end, it is without a doubt that I did learn more, and the knowledge I have been able to take with me includes a wider spectrum than the topic of population health and medication utilization. -

Supplementary Material
Supplementary material Table S1. Search strategy performed on the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL). 1. Randomi*ed study OR random allocation OR Randomi*ed controlled trial OR Random* Control* trial OR RCT Epidemiological study 2. sodium glucose cotransporter 2 OR sodium glucose cotransporter 2 inhibitor* OR sglt2 inhibitor* OR empagliflozin OR dapagliflozin OR canagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR sotagliflozin OR sergliflozin OR remogliflozin 3. 1 AND 2 1 Table S2. Safety outcomes of empagliflozin and linagliptin combination therapy compared with empagliflozin or linagliptin monotherapy in treatment naïve type 2 diabetes patients Safety outcome Comparator 1 Comparator 2 I2 RR [95% CI] Number of events Number of events / / total subjects total subjects i. Empagliflozin + linagliptin vs empagliflozin monotherapy Empagliflozin + Empagliflozin linagliptin monotherapy ≥ 1 AE(s) 202/272 203/270 77% 0.99 [0.81, 1.21] ≥ 1 drug-related 37/272 38/270 0% 0.97 [0.64, 1.47] AE(s) ≥ 1 serious AE(s) 13/272 19/270 0% 0.68 [0.34, 1.35] Hypoglycaemia* 0/272 5/270 0% 0.18 [0.02, 1.56] UTI 32/272 25/270 29% 1.28 [0.70, 2.35] Events suggestive 12/272 13/270 9% 0.92 [0.40, 2.09] of genital infection i. Empagliflozin + linagliptin vs linagliptin monotherapy Empagliflozin + Linagliptin linagliptin monotherapy ≥ 1 AE(s) 202/272 97/135 0% 1.03 [0.91, 1.17] ≥ 1 drug-related 37/272 17/135 0% 1.08 [0.63, 1.84] AE(s) ≥ 1 serious AE(s) 13/272 2/135 0% 3.22 [0.74, 14.07] Hypoglycaemia* 0/272 1/135 NA 0.17 [0.01, 4.07] UTI 32/272 12/135 0% 1.32 [0.70, 2.49] Events suggestive 12/272 4/135 0% 1.45 [0.47, 4.47] of genital infection RR, relative risk; AE, adverse event; UTI, urinary tract infection. -

A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D
Drugs 2005; 65 (3): 325-340 REVIEW ARTICLE 0012-6667/05/0003-0325/$39.95/0 2005 Adis Data Information BV. All rights reserved. A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D. Mooradian Division of Endocrinology, Department of Internal Medicine, Diabetes, and Metabolism, St Louis University School of Medicine, St Louis, Missouri, USA Contents Abstract ....................................................................................325 1. Physiology of Insulin Secretion .............................................................326 2. Conventional Insulin Preparations ..........................................................327 3. Insulin Analogues ........................................................................328 3.1 Rapid-Acting Insulin Analogues .......................................................328 3.1.1 Insulin Lispro ...................................................................328 3.1.2 Insulin Aspart ..................................................................329 3.1.3 Insulin Glulisine .................................................................329 3.1.4 Clinical Utility of Rapid-Acting Insulin Analogues ...................................330 3.2 Premixed Insulins and Insulin Analogues ................................................331 3.3 Basal Insulin Analogues ...............................................................331 3.3.1 Insulin Glargine ................................................................331 -

IHS PROVIDER September 2017
September 2017 Volume 42 Number 9 Indian Health Service National Pharmacy and Therapeutics Committee SGLT-2 inhibitors (Update) NPTC Formulary Brief August Meeting 2017 Background: The FDA has currently approved three SGLT-2 inhibitors, two of which have completed FDA-mandated cardiovascular outcomes trials. Last year, in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG), empagliflozin not only reduced cardiovascular events, but also mortality.1 This year, the Canagliflozin Cardiovascular Assessment Study (CANVAS) demonstrated equivocal cardiovascular benefits, no mortality benefit, and significant harms in those receiving canagliflozin.2 The DECLARE-TIMI 58 cardiovascular study of dapagliflozin will be completed in April 2019 (ClinicalTrials.gov Identifier: NCT01730534). Uncertainty remains regarding the current data, long- term benefits and harms, and differentiation among SGLT-2 inhibitors. Following a review of SGLT2 inhibitors at the August 2017 NPTC meeting on their cardiovascular outcomes, net benefit and place in therapy, no modifications were made to the National Core Formulary (NCF). Discussion: EMPA-REG enrolled 7,020 patients with Type 2 diabetes mellitus (T2DM) and HgbA1c values between 7.0-10.0%. All patients had established cardiovascular disease (CVD) and were observed for a median duration of 3.1 years. Empagliflozin reduced the primary outcome of cardiovascular (CV) death, nonfatal myocardial infarction (MI), or nonfatal cardiovascular accident (CVA) by 6.5 events per 1000 patient-years (pt-yrs). Mortality decreased by 9.2 events per 1000 pt-yrs, primarily driven by a reduction in CV mortality of 7.8 events per 1000 pt-yrs. Heart failure hospitalization decreased by 5.1 events per 1000 pt-yrs. -
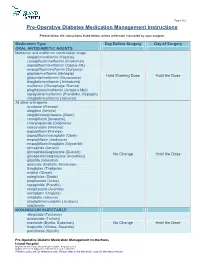
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -

Effects of Basal Insulin Analog and Metformin on Glycaemia Control and Weight As Risk &Factors for Endothelial Dysfunction
EFFECTS OF BASAL INSULIN ANALOG AND METFORMIN ON GLYCAEMIA CONTROL AND WEIGHT AS RISK &FACTORS FOR ENDOTHELIAL DYSFUNCTION Belma Aščić – Buturović¹*, Mirsad Kacila² ¹ Clinic of Endocrinology, Diabetes Mellitus and Metabolic Diseases, University of Sarajevo Clinics Centre, Bolnička , Sarajevo, Bosnia and Herzegovina ² Center for Heart Diseases, University of Sarajevo Clinics Centre, Bolnička , Sarajevo, Bosnia and Herzegovina * Corresponding author Abstract Obese patients with type diabetes and impaired glucose tolerance are at increased risk of develop- ment of cardiovascular diseases. Endothelial dysfunction may be a reason for development of ath- erosclerosis and cardiovascular diseases. Lifestyle modifi cation, increased physical activity, weight reduction, energy restricted diet and good glycaemia control can be useful for the endothelial func- tion improvement and may decrease the risk of cardiovascular diseases. Th e aim of this study was to evaluate the eff ects of basal insulin analog and metformin on glycaemia control and weight as risk factors of endothelial dysfunction. Total of patients ( male and female) with type dia- betes were studied. Th e patients were monitored over six months period. Glycated hemoglobin (HbAc), fasting plasma glucose (FPG), postprandial plasma glucose (PPG), and body mass index (BMI) were observed. Mean age of the subjects was , ± , years. Mean diabetes duration was , ± , years. At the end of the study mean body mass index decreased from , ± , kg/m to , ±, kg/m. In this study we included diabetic patients with fasting glycaemia over mmol/ dm, postmeal glycaemia over , mmol/dm and glycated hemoglobin over . Prior to the study, the patients were treated with premix insulin divided in two daily doses and metformin after the lunch, which did not result in suffi cient regulation of glycaemia. -

NESINA (Alogliptin) Tablets, for Oral Use • Heart Failure: Consider the Risks and Benefits of NESINA Prior to Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use • Acute pancreatitis: There have been postmarketing reports of NESINA safely and effectively. See full prescribing information for acute pancreatitis. If pancreatitis is suspected, promptly NESINA. discontinue NESINA. (5.1) NESINA (alogliptin) tablets, for oral use • Heart failure: Consider the risks and benefits of NESINA prior to Initial U.S. Approval: 2013 initiating treatment in patients at risk for heart failure. If heart failure develops, evaluate and manage according to current ---------------------------RECENT MAJOR CHANGES-------------------------- standards of care and consider discontinuation of NESINA (5.2). Indications and Usage (1.1) 4/2016 • Hypersensitivity: There have been postmarketing reports of Dosage and Administration serious hypersensitivity reactions in patients treated with NESINA Patients with Renal Impairment (2.2) 4/2016 such as anaphylaxis, angioedema and severe cutaneous adverse Warnings and Precautions reactions, including Stevens-Johnson syndrome. In such cases, Pancreatitis (5.1) 4/2016 promptly discontinue NESINA, assess for other potential causes, Heart Failure (5.2) 4/2016 institute appropriate monitoring and treatment and initiate Hepatic Effects (5.4) 4/2016 alternative treatment for diabetes. (5.3) Bullous Pemphigoid (5.7) 12/2016 • Hepatic effects: Postmarketing reports of hepatic failure, ----------------------------INDICATIONS AND USAGE--------------------------- sometimes fatal. Causality cannot be excluded. If liver injury is NESINA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an detected, promptly interrupt NESINA and assess patient for adjunct to diet and exercise to improve glycemic control in adults with probable cause, then treat cause if possible, to resolution or type 2 diabetes mellitus. -

Diabetes Recommendations and Tier Coverage Chart
DIABETES RECOMMENDATIONS AND TIER COVERAGE CHART The American Diabetes Association guidelines for 2020, recommend metformin as the preferred initial treatment for type 2 diabetes (T2DM) along with weight management and physical activity. In patients who have established ASVD or at high risk, CKD, or HF, a SGLT2i or GLP-1 receptor with proven efficacy is recommended independent of A1C. • ASCVD dominates: o GLP-1RA with proven CVD benefit (dulaglutide, liraglutide, injectable semaglutide) OR o SGLT2i with proven CVD benefit (canagliflozin, empagliflozin) if adequate eGFR • HF or CKD dominates: o SGLT2i with evidence of reducing HF and/or CKD progression (empagliflozin, canagliflozin, dapagliflozin) if adequate eGFR OR o If SGLT2i intolerant/contraindicated or eGFR is inadequate, then GLP-1RA with proven CVD benefit In individuals without established cardiovascular disease, pharmacological treatment should be patient-centered taking into account side-effects, cost, impact on weight, risk of hypoglycemia, and other patient preferences. For more detailed information regarding ADA recommendations for pharmacological agents to treat T2DM click here. The following chart is a list of oral and injectable diabetes medications listed by class with their respective A1C reduction and insurance coverage and/or coverage requirements for BCBS, HPHC, Tufts, TMP, and MassHealth. Tufts Medicare Medications BCBSMA HPHC Tufts Preferred MassHealth Biguanides A1C reduction: 1-1.5% metformin Tier 1 Tier 1;2 Tier 1 Tier 1 Covered Glucoghage (metformin) NC NC NC;Tier