ANTI-HYPERGLYCEMIC DIABETES AGENTS in T2DM: Color Outcomes Comparison Summary Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment of Diabetes Mellitus
TREATMENT OF DIABETES MELLITUS DIABETES is a condition that affects how the body makes energy from food. Food is broken down into sugar (glucose) in the body and released into the blood. When the blood sugar level rises after a meal, insulin responds to let the sugar into the cells to be used as energy. In diabetes, the body either does not make enough insulin or it stops responding to insulin as well as it should. This results in sugar staying in the blood and leads to serious health problems over time. DIAGNOSIS OF DIABETES1 • A1C Test: Lab test measuring average blood sugar over past two to three months • Fasting Blood Sugar Test: Lab test measuring blood sugar after eight hours of no food or drink • Oral Glucose Tolerance Test (OGTT): Measures blood sugar before and two hours after drinking a specific sugary liquid • Random Blood Sugar Test: Measures blood sugar at a moment in time, without any kind of preparation (like fasting) FASTING BLOOD ORAL GLUCOSE TOLERANCE RANDOM BLOOD RESULT A1C TEST SUGAR TEST TEST SUGAR TEST Diabetes ≥ 6.5% ≥126 mg/dL ≥ 200 mg/dL ≥ 200 mg/dL Prediabetes 5.7 – 6.4% 100 – 125 mg/dL 140 – 199 mg/dL N/A Normal < 5.7% ≤99 mg/dL < 140 mg/dL N/A NON-DRUG TREATMENTS2 THERAPY COST WHAT TO EXPECT Diet (Mediterranean diet) and exercise (30 minutes a day, five days a week of moderate- Weight loss $-$$ intensity exercise); 7% weight loss decreases risk of diabetes3 Psychological intervention $$-$$$ Psychotherapy may reduce diabetic distress and improve glycemic control4,5 nationalcooperativerx.com PRESCRIPTION TREATMENTS -

Think Medicines!
Think Issue 13 August 2016 MHRA Drug Safety SGLT2 inhibitors: updated advice on the risk of diabetic Medicines!Updates can be found ketoacidosis (DKA) at the following link. The MHRA are advising health care professionals to test for raised ketones in patients with ketoacidosis symptoms, who are taking SGLT2 inhibitors even if New higher strength Humalog® plasma glucose levels are near-normal. SGLT2 inhibitors include: Canagliflozin, 200 units/ml KwikPen™ Dapagliflozin and Empagliflozin. Serious, life-threatening, and fatal cases of In order to minimize medication DKA have been reported in patients taking an SGLT2 inhibitor. In several cases, errors. blood glucose levels were only moderately elevated (e.g. <14mmol/L) Insulin lispro 200 units/ml representing an atypical presentation for DKA, which could delay diagnosis and solution for injection should treatment. ONLY be administered using the Advice for health Care Professionals: Humalog 200 units/ml pre-filled Inform patients of the signs of diabetic ketoacidosis (DKA) and advise them to pen (KwikPen). seek immediate medical advice if they develop any of these symptoms (e.g. When switching from one rapid weight loss, feeling sick or being sick, stomach pain, fast and deep Humalog strength to another, breathing, sleepiness, a sweet smell to the breath, a sweet or metallic taste in the dose does not need to be the mouth, or a different odour to urine or sweat). converted. Unnecessary dose Discuss the risk factors of DKA with patients. conversion may lead to under/ Discontinue treatment with the SGLT2 inhibitor immediately if DKA is over dosing and resultant hyper/ suspected or diagnosed. -

Supplementary Material
Supplementary material Table S1. Search strategy performed on the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL). 1. Randomi*ed study OR random allocation OR Randomi*ed controlled trial OR Random* Control* trial OR RCT Epidemiological study 2. sodium glucose cotransporter 2 OR sodium glucose cotransporter 2 inhibitor* OR sglt2 inhibitor* OR empagliflozin OR dapagliflozin OR canagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR sotagliflozin OR sergliflozin OR remogliflozin 3. 1 AND 2 1 Table S2. Safety outcomes of empagliflozin and linagliptin combination therapy compared with empagliflozin or linagliptin monotherapy in treatment naïve type 2 diabetes patients Safety outcome Comparator 1 Comparator 2 I2 RR [95% CI] Number of events Number of events / / total subjects total subjects i. Empagliflozin + linagliptin vs empagliflozin monotherapy Empagliflozin + Empagliflozin linagliptin monotherapy ≥ 1 AE(s) 202/272 203/270 77% 0.99 [0.81, 1.21] ≥ 1 drug-related 37/272 38/270 0% 0.97 [0.64, 1.47] AE(s) ≥ 1 serious AE(s) 13/272 19/270 0% 0.68 [0.34, 1.35] Hypoglycaemia* 0/272 5/270 0% 0.18 [0.02, 1.56] UTI 32/272 25/270 29% 1.28 [0.70, 2.35] Events suggestive 12/272 13/270 9% 0.92 [0.40, 2.09] of genital infection i. Empagliflozin + linagliptin vs linagliptin monotherapy Empagliflozin + Linagliptin linagliptin monotherapy ≥ 1 AE(s) 202/272 97/135 0% 1.03 [0.91, 1.17] ≥ 1 drug-related 37/272 17/135 0% 1.08 [0.63, 1.84] AE(s) ≥ 1 serious AE(s) 13/272 2/135 0% 3.22 [0.74, 14.07] Hypoglycaemia* 0/272 1/135 NA 0.17 [0.01, 4.07] UTI 32/272 12/135 0% 1.32 [0.70, 2.49] Events suggestive 12/272 4/135 0% 1.45 [0.47, 4.47] of genital infection RR, relative risk; AE, adverse event; UTI, urinary tract infection. -

Steglujan, INN-Ertugliflozin-Sitagliptin
25 January 2018 EMA/86941/2018 Committee for Medicinal Products for Human Use (CHMP) Assessment report Steglujan International non-proprietary name: ertugliflozin / sitagliptin Procedure No. EMEA/H/C/004313/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 7 1.1. Submission of the dossier ...................................................................................... 7 1.2. Steps taken for the assessment of the product ......................................................... 8 2. Scientific discussion ................................................................................ 9 2.1. Problem statement ............................................................................................... 9 2.1.1. Disease or condition ........................................................................................... 9 2.1.2. Epidemiology .................................................................................................... 9 2.1.3. Clinical presentation .......................................................................................... -

IHS PROVIDER September 2017
September 2017 Volume 42 Number 9 Indian Health Service National Pharmacy and Therapeutics Committee SGLT-2 inhibitors (Update) NPTC Formulary Brief August Meeting 2017 Background: The FDA has currently approved three SGLT-2 inhibitors, two of which have completed FDA-mandated cardiovascular outcomes trials. Last year, in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG), empagliflozin not only reduced cardiovascular events, but also mortality.1 This year, the Canagliflozin Cardiovascular Assessment Study (CANVAS) demonstrated equivocal cardiovascular benefits, no mortality benefit, and significant harms in those receiving canagliflozin.2 The DECLARE-TIMI 58 cardiovascular study of dapagliflozin will be completed in April 2019 (ClinicalTrials.gov Identifier: NCT01730534). Uncertainty remains regarding the current data, long- term benefits and harms, and differentiation among SGLT-2 inhibitors. Following a review of SGLT2 inhibitors at the August 2017 NPTC meeting on their cardiovascular outcomes, net benefit and place in therapy, no modifications were made to the National Core Formulary (NCF). Discussion: EMPA-REG enrolled 7,020 patients with Type 2 diabetes mellitus (T2DM) and HgbA1c values between 7.0-10.0%. All patients had established cardiovascular disease (CVD) and were observed for a median duration of 3.1 years. Empagliflozin reduced the primary outcome of cardiovascular (CV) death, nonfatal myocardial infarction (MI), or nonfatal cardiovascular accident (CVA) by 6.5 events per 1000 patient-years (pt-yrs). Mortality decreased by 9.2 events per 1000 pt-yrs, primarily driven by a reduction in CV mortality of 7.8 events per 1000 pt-yrs. Heart failure hospitalization decreased by 5.1 events per 1000 pt-yrs. -

Step Therapy
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2020 P 3086-9 Program Step Therapy – Diabetes Medications - SGLT2 Inhibitors Medication Farxiga (dapagliflozin)*, Glyxambi (empagliflozin/linagliptan), Invokana (canagliflozin)*, Invokamet (canagliflozin/metformin)*, Invokamet XR (canaglifloxin/metformin extended-release)*, Jardiance (empagliflozin), Qtern (dapagliflozin/saxagliptin)*, Segluromet (ertugliflozin/metformin)*, Steglatro (ertugliflozin)*, Steglujan (ertugliflozin/sitagliptin)*, Xigduo XR (dapagliflozin/metformin extended-release)* P&T Approval Date 10/2016, 10/2017, 4/2018, 8/2018, 12/2018, 2/2019, 2/2020, 5/2020; 7/2020 Effective Date 10/1/2020; Oxford only: 10/1/2020 1. Background: Farxiga (dapagliflozin)*, Invokana (canagliflozin)*, Jardiance (empagliflozin) and Steglatro (ertugliflozin)* are sodium-glucose co-transporter 2 (SGLT2) inhibitors indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Farxiga*, Invokana* and Jardiance have additional indications. Farxiga* is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure (NYHA class II-IV) with reduced ejection fraction. Invokana* is indicated to reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD), and to reduce the risk of end-stage kidney disease (ESKD), doubling of -

Steglujan (Ertugliflozin/Sitagliptin), Segluromet
Steglatro™ (ertugliflozin), Steglujan™ (ertugliflozin/sitagliptin), Segluromet™ (ertugliflozin/metformin) – New drug approval • On December 19, 2017, the FDA approved Merck’s Steglatro (ertugliflozin), Steglujan (ertugliflozin/sitagliptin), and Segluromet (ertugliflozin/metformin) as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM). — Steglujan is indicated when treatment with both ertugliflozin and sitagliptin is appropriate. — Segluromet is indicated when patients are not adequately controlled on a regimen containing ertugliflozin or metformin, or in patients who are already treated with both ertugliflozin and metformin. — These drugs are not recommended in patients with type 1 diabetes mellitus (T1DM) or for the treatment of diabetic ketoacidosis. — Steglujan has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using Steglujan. • Ertugliflozin is the fourth sodium-glucose co-transoporter-2 (SGLT2) inhibitor approved by the FDA. Other marketed SGLT2 inhibitors include Jardiance® (empagliflozin), Farxiga® (dapagliflozin), and Invokana® (canagliflozin). • The efficacy and safety of ertugliflozin were evaluated in multiple clinical studies involving patients with T2DM. Ertugliflozin was studied as monotherapy and in combination with metformin and/or a dipeptidyl peptidase 4 (DPP-4) inhibitor. Ertugliflozin was also studied in combination with other antidiabetic medications, including insulin and a sulfonylurea, and in T2DM patients with moderate renal impairment. — In patients with T2DM, treatment with ertugliflozin, ertugliflozin + sitagliptin, and ertugliflozin + metformin reduced hemoglobin A1c (HbA1c) vs. placebo or the active comparator. — In patients with T2DM and moderate renal impairment, treatment with ertugliflozin did not result in a reduction in HbA1c vs. -

Newer Diabetes Treatments Drug Class Update with New Drug Evaluation: Semaglutide and Ertugliflozin
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Newer Diabetes Treatments Drug Class Update with New Drug Evaluation: Semaglutide and Ertugliflozin Date of Review: July 2018 Date of Last Review: September 2017 End Date of Literature Search: 05/23/2018 Generic Name: semaglutide Brand Name (Manufacturer): Ozempic® (Novo Nordisk) Generic Name: ertugliflozin, ertugliflozin/sitagliptin, ertugliflozin/metformin Brand Name (Manufacturer): Steglatro™, Steglujan™, Segluromet™ (Merck & Co., Inc.) Dossier Received: ertugliflozin (yes), semaglutide (no) Current Status of PDL Class: See Appendix 1. Purpose for Class Update: To evaluate the safety and efficacy of semaglutide and ertugliflozin (and combinations) which were recently approved for blood glucose lowering in patients with type 2 diabetes mellitus (T2DM). High quality new evidence published since the last review will also be presented. Research Questions: 1. In patients with T2DM, is there any new comparative evidence for non-insulin antidiabetic therapies based on surrogate efficacy outcomes (e.g., hemoglobin A1c [HbA1c]) and long-term clinically meaningful effectiveness outcomes (e.g., microvascular outcomes, macrovascular outcomes and mortality)? 2. In patients with T2DM, is there any new comparative evidence for non-insulin diabetes treatments based on harms outcomes (e.g., severe hypoglycemia, heart failure, diabetic ketoacidosis, pancreatitis, etc.)? 3. Are there subpopulations of patients with T2DM for which specific therapies may be more effective or associated with less harm? 4. What are the efficacy and harms evidence for the two new non-insulin diabetes treatments, ertugliflozin and semaglutide? Conclusions: A Drug Effectiveness Review Project (DERP) update on newer diabetes therapies, three new guidelines/standards, one new randomized controlled trial and two new drug reviews were reviewed for this class update. -
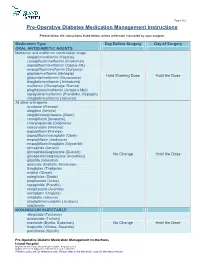
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

Ertugliflozin 5Mg, 15Mg Film-Coated Tablet (Steglatro®) Merck Sharp & Dohme
1 www.scottishmedicines.org.uk SMC2102 ertugliflozin 5mg, 15mg film-coated tablet (Steglatro®) Merck Sharp & Dohme 7 December 2018 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards and Area Drug and Therapeutic Committees (ADTCs) on its use in NHSScotland. The advice is summarised as follows: ADVICE: following a full submission ertugliflozin (Steglatro®) is accepted for restricted use within NHSScotland. Indication under review: in adults aged 18 years and older with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control: As monotherapy in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications. In addition to other medicinal products for the treatment of diabetes. SMC restriction: ertugliflozin is accepted for use as monotherapy and as add-on therapy. When used as monotherapy it is restricted to patients who would otherwise receive a dipeptidyl peptidase-4 inhibitor and in whom a sulphonylurea or pioglitazone is not appropriate. Ertugliflozin was superior to placebo in lowering HbA1c in adults with type 2 diabetes mellitus in phase III studies in monotherapy, dual therapy and triple therapy settings. Chairman Scottish Medicines Consortium Published 14 January 2019 1 Indication In adults aged 18 years and older with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control: As monotherapy in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications. In addition to other medicinal products for the treatment of diabetes.1 Dosing Information The recommended starting dose is 5mg orally once daily which can, if tolerated, be increased to 15mg once daily if additional glycaemic control is needed. -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

The Sodium Glucose Cotransporter Type 2 Inhibitor Empagliflozin Preserves B-Cell Mass and Restores Glucose Homeostasis in the Male Zucker Diabetic Fatty Rat
1521-0103/350/3/657–664$25.00 http://dx.doi.org/10.1124/jpet.114.213454 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 350:657–664, September 2014 Copyright ª 2014 by The American Society for Pharmacology and Experimental Therapeutics The Sodium Glucose Cotransporter Type 2 Inhibitor Empagliflozin Preserves b-Cell Mass and Restores Glucose Homeostasis in the Male Zucker Diabetic Fatty Rat Henrik H. Hansen, Jacob Jelsing, Carl Frederik Hansen, Gitte Hansen, Niels Vrang, Michael Mark, Thomas Klein, and Eric Mayoux Gubra, Hørsholm, Denmark (H.H.H., J.J., C.F.H., G.H., N.V.); and Boehringer Ingelheim Pharma, Biberach, Germany (M.M., T.K., E.M.) Received January 22, 2014; accepted July 2, 2014 Downloaded from ABSTRACT Type 2 diabetes is characterized by impaired b-cell function at week 8 of treatment compared with week 4, those of empagliflozin associated with progressive reduction of insulin secretion and remained stable throughout the study period. Similarly, empagliflozin b -cell mass. Evidently, there is an unmet need for treatments improved glucose tolerance and preserved insulin secretion jpet.aspetjournals.org with greater sustainability in b-cell protection and antidiabetic after both 4 and 8 weeks of treatment. These effects were efficacy. Through an insulin and b cell–independent mechanism, reflected by less reduction in b-cell mass with empagliflozin or empagliflozin, a specific sodium glucose cotransporter type 2 liraglutide at week 4, whereas only empagliflozin showed b-cell (SGLT-2) inhibitor, may potentially provide longer efficacy. This sparing effects also at week 8. Although this study cannot be study compared the antidiabetic durability of empagliflozin treat- used to dissociate the absolute antidiabetic efficacy among ment (10 mg/kg p.o.) against glibenclamide (3 mg/kg p.o.) and the different mechanisms of drug action, the study demon- liraglutide (0.2 mg/kg s.c.) on deficient glucose homeostasis and strates that empagliflozin exerts a more sustained improve- b-cell function in Zucker diabetic fatty (ZDF) rats.