A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidenz Und Versorgungsrealität Von Kurzwirksamen Insulinanaloga in Der Behandlung Des Typ-2-Diabetes Mellitus
Evidenz und Versorgungsrealität von kurzwirksamen Insulinanaloga in der Behandlung des Typ-2-Diabetes mellitus – Eine Versorgungsanalyse auf der Basis von Sekundärdaten – Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften vorgelegt beim Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität in Frankfurt am Main von Matthias S. Pfannkuche aus Brühl Frankfurt am Main, im Jahr 2009 vom Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität als Dissertation angenommen. Dekan: Prof. Dr. rer. nat. Dieter Steinhilber Gutachter: Prof. Dr. rer. nat. Theo Dingermann Prof. Dr. rer. nat. Gerd Glaeske (Universität Bremen) Datum der Disputation: 15. Februar 2010 Bildnachweis Titelseite: Insulin Hexamer: http://commons.wikimedia.org/wiki/File:Human-insulin-hexamer-3D-ribbons.png (letzter Zugriff: 11.06.2009) Non semper ea sunt, quae videntur. (Phädrus, fabulae 4, 2, 5) Danksagung Diese Dissertation sowie die hiermit in Verbindung stehenden Publikationen wären ohne die Anregungen und Unterstützung durch viele Kollegen, Freunde und Organisationen nicht möglich gewesen. Ihnen möchte ich an dieser Stelle danken. Mein besonderer Dank gilt Prof. Dr. rer. nat Gerd Glaeske und Prof. Dr. rer. nat. Theo Dingermann, die diese Arbeit in vielerlei Hinsicht erst ermöglichten. Überaus dankbar bin ich Prof. Dr. rer. nat. Gerd Glaeske für die freundliche Aufnahme in seine Arbeitsgruppe, die es mir ermöglichte weitere Einblicke in die Gesundheitsökonomie, Gesundheitspolitik und Versorgungsforschung zu nehmen. Für das Korrekturlesen der kompletten Arbeit, die zahlreichen Hinweise und konstruktiven Diskussionen sowie die zahlreichen Mittagspausen danke ich im besonderen Dr. P.H. Falk Hoffmann. Herzlicher Dank gilt auch dem gesamten Arbeitskreis in Bremen sowie den Projektbeteiligten Krankenkassen, allen voran der GEK, die mir durch den Zugriff auf ihre Daten erst viele Analysen ermöglichten. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Insulin Aspart Sanofi, If It Is Coloured Or It Has Solid Pieces in It
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT Insulin aspart Sanofi 100 units/ml solution for injection in vial Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg). Insulin aspart Sanofi 100 units/ml solution for injection in vial Each vial contains 10 ml equivalent to 1,000 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Each cartridge contains 3 ml equivalent to 300 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen Each pre-filled pen contains 3 ml equivalent to 300 units insulin aspart. Each pre-filled pen delivers 1-80 units in steps of 1 unit. *produced in Escherichia coli by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection (injection). Clear, colourless, aqueous solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Insulin aspart Sanofi is indicated for the treatment of diabetes mellitus in adults, adolescents and children aged 1 year and above. 4.2 Posology and method of administration Posology The potency of insulin analogues, including insulin aspart, is expressed in units, whereas the potency of human insulin is expressed in international units. -

Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba
Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba by Kim¡ T. G. Guilbert A Thesis submitted to The Faculty of Graduate Studies in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Faculty of Pharmacy The University of Manitoba Winnipeg, Manitoba @ Kimi T.G. Guilbert, March 2005 TIIE UMYERSITY OF MANITOBA F'ACULTY OF GRADUATE STTJDIES +g+ù+ COPYRIGIIT PERMISSION PAGE Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba BY Kimi T.G. Guilbert A ThesisÆracticum submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfillment of the requirements of the degree of MASTER OF SCIENCE KIMI T.G. GTIILBERT O2()O5 Permission has been granted to the Library of The University of Manitoba to lend or sell copies of this thesis/practicum, to the National Library of Canada to microfïlm this thesis and to lend or sell copies of the film, and to University Microfilm Inc. to publish an abstract of this thesis/practicum. The author reserves other publication rights, and neither this thesis/practicum nor extensive extracts from it may be printed or otherwise reproduced without the author's written permission. Acknowledgements Upon initiation of this project I had a clear objective in mind--to learn more. As with many endeavors in life that are worthwhile, the path I have followed has brought me many places I did not anticipate at the beginning of my journey. ln reaching the end, it is without a doubt that I did learn more, and the knowledge I have been able to take with me includes a wider spectrum than the topic of population health and medication utilization. -

(Pram) and Insulin A21G Improves Post-Prandial Glucose Vs Novolog
ADO09, A Co-Formulation Of Pramlintide (Pram) and Insulin A21G improves Post-Prandial Glucose Vs Novolog® in Type 1 Diabetes (T1DM) G.Meiffren¹, G.Andersen², R.Eloy¹, C.Seroussi¹, C.Mégret¹, S.Famulla², Y.-P Chan¹, M.Gaudier¹, O.Soula¹, J.H. DeVries²,T.Heise² (1 Adocia, Lyon, France ; 2 Profil, Neuss, Germany) Introduction & Background Overall safety Outpatient period results - CGM metrics o ADO09 (M1Pram) is a co-formulation of pramlintide and insulin A21G o Both treatments were well tolerated without any treatment-related serious adverse events o Most of the CGM metrics (TiR [70-180], TiR [80-140], mean blood glucose per day), were significantly improved developed to leverage the beneficial effects of pramlintide on post-prandial (Table 2). As expected M1Pram had numerically more, mostly gastrointestinal adverse events with M1Pram (Table 4). Postprandial and mean 24-hour glucose profiles were improved with M1Pram (Fig. 3) glucose without additional injections than insulin aspart Table 4: CGM metrics, all days. Significant differences are marked in bold Objective and design o No severe hypoglycemia were seen, slightly more hypoglycemic events occurred with M1Pram Ratio of LSMean* o To compare the effect of M1Pram and insulin aspart (Novolog®, Novo than with aspart (Table 3) Difference Parameter Treatment LS Mean M1Pram / Aspart P-value Nordisk) on post-prandial glucose control, glycemic control assessed by Table 2: Incidence of adverse events throughout the trial (M1Pram-Aspart) (95% CI) CGM and safety/tolerability M1Pram Aspart M1Pram -

IHS PROVIDER September 2017
September 2017 Volume 42 Number 9 Indian Health Service National Pharmacy and Therapeutics Committee SGLT-2 inhibitors (Update) NPTC Formulary Brief August Meeting 2017 Background: The FDA has currently approved three SGLT-2 inhibitors, two of which have completed FDA-mandated cardiovascular outcomes trials. Last year, in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG), empagliflozin not only reduced cardiovascular events, but also mortality.1 This year, the Canagliflozin Cardiovascular Assessment Study (CANVAS) demonstrated equivocal cardiovascular benefits, no mortality benefit, and significant harms in those receiving canagliflozin.2 The DECLARE-TIMI 58 cardiovascular study of dapagliflozin will be completed in April 2019 (ClinicalTrials.gov Identifier: NCT01730534). Uncertainty remains regarding the current data, long- term benefits and harms, and differentiation among SGLT-2 inhibitors. Following a review of SGLT2 inhibitors at the August 2017 NPTC meeting on their cardiovascular outcomes, net benefit and place in therapy, no modifications were made to the National Core Formulary (NCF). Discussion: EMPA-REG enrolled 7,020 patients with Type 2 diabetes mellitus (T2DM) and HgbA1c values between 7.0-10.0%. All patients had established cardiovascular disease (CVD) and were observed for a median duration of 3.1 years. Empagliflozin reduced the primary outcome of cardiovascular (CV) death, nonfatal myocardial infarction (MI), or nonfatal cardiovascular accident (CVA) by 6.5 events per 1000 patient-years (pt-yrs). Mortality decreased by 9.2 events per 1000 pt-yrs, primarily driven by a reduction in CV mortality of 7.8 events per 1000 pt-yrs. Heart failure hospitalization decreased by 5.1 events per 1000 pt-yrs. -

Effects of Exenatide on Cardiac Function, Perfusion, and Energetics
Chen et al. Cardiovasc Diabetol (2017) 16:67 DOI 10.1186/s12933-017-0549-z Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine Weena J. Y. Chen1*, Michaela Diamant1^, Karin de Boer2, Hendrik J. Harms3, Lourens F. H. J. Robbers2, Albert C. van Rossum2, Mark H. H. Kramer1, Adriaan A. Lammertsma3 and Paul Knaapen2 Abstract Background: Multiple bloodglucose-lowering agents have been linked to cardiovascular events. Preliminary studies showed improvement in left ventricular (LV) function during glucagon-like peptide-1 receptor agonist administration. Underlying mechanisms, however, are unclear. The purpose of this study was to investigate myocardial perfusion and oxidative metabolism in type 2 diabetic (T2DM) patients with LV systolic dysfunction as compared to healthy controls. Furthermore, efects of 26-weeks of exenatide versus insulin glargine administration on cardiac function, perfusion and oxidative metabolism in T2DM patients with LV dysfunction were explored. Methods and results: Twenty-six T2DM patients with LV systolic dysfunction (cardiac magnetic resonance (CMR) derived LV ejection fraction (LVEF) of 47 13%) and 10 controls (LVEF of 59 4%, P < 0.01 as compared to patients) were analyzed. Both myocardial perfusion± during adenosine-induced hyperemia± (P < 0.01), and coronary fow reserve 15 (P < 0.01), measured by [ O]H2O positron emission tomography (PET), were impaired in T2DM patients as compared to healthy controls. Myocardial oxygen consumption and myocardial efciency, measured using [11C]acetate PET and CMR derived stroke volume, were not diferent between the groups. -

OZEMPIC (Semaglutide) Injection, for Subcutaneous Use Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙CONTRAINDICATIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ These highlights do not include all the information needed to use Personal or family history of medullary thyroid carcinoma or in patients OZEMPIC® safely and effectively. See full prescribing information with Multiple Endocrine Neoplasia syndrome type 2 (4). for OZEMPIC. Known hypersensitivity to OZEMPIC or any of the product components (4). OZEMPIC (semaglutide) injection, for subcutaneous use Initial U.S. Approval: 2017 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙WARNINGS AND PRECAUTIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ Pancreatitis: Has been reported in clinical trials. Discontinue promptly if WARNING: RISK OF THYROID C-CELL TUMORS pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2). See full prescribing information for complete boxed warning. Diabetic Retinopathy Complications: Has been reported in a clinical trial. Patients with a history of diabetic retinopathy should be monitored (5.3). In rodents, semaglutide causes thyroid C-cell tumors. It is Never share an OZEMPIC pen between patients, even if the needle is unknown whether OZEMPIC causes thyroid C-cell tumors, changed (5.4). including medullary thyroid carcinoma (MTC), in humans as Hypoglycemia: When OZEMPIC is used with an insulin secretagogue or the human relevance of semaglutide-induced rodent thyroid C- insulin, consider lowering the dose of the secretagogue or insulin to reduce cell tumors has not been determined (5.1, 13.1). the risk of hypoglycemia (5.5). OZEMPIC is contraindicated in patients with a personal or Acute Kidney Injury: Monitor renal function in patients with renal family history of MTC or in patients with Multiple Endocrine impairment reporting severe adverse gastrointestinal reactions (5.6). Neoplasia syndrome type 2 (MEN 2). -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -
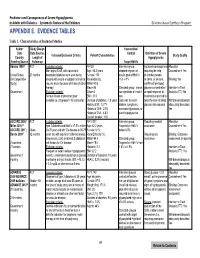
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-Based Synthesis Program APPENDIX E
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-based Synthesis Program APPENDIX E. EVIDENCE TABLES Table 1. Characteristics of Included Studies Author Study Design Intervention/ Date Data Sources Control Definition of Severe Inclusion/Exclusion Criteria Patient Characteristics Study Quality Country Length of Hypoglycemia Funding Source Follow-up Target HbA1c Abraira 199530 RCT Inclusion criteria: N=153 Intensive group: Impaired consciousness Allocation Men ages 40-69, with non-insulin Age: 60.2 years stepped regimen of requiring the help Concealment: Yes United States 27 months dependent diabetes who were being % male: 100 insulin goal of HbA1c of another person, (VA Cooperative treated with insulin or judged clinically to Race/ethnicity: =5.1+/-1% or coma, or seizure; Blinding: Yes Study) require insulin because of failure of other White=49.5 confirmed low blood therapy Black=24 Standard group: one or glucose concentration Intention-to-Treat Government Exclusion criteria: Other=3 two injections of insulin/ or rapid response to Analysis (ITT): No Serious illness or predicted poor BMI: 31.0 day treatments expected to compliance, diagnosed >15 years prior Duration of diabetes: 7.8 years Goal was to avoid raise the level of blood Withdrawals/dropouts History of MI: 13.7% diabetic symptoms, glucose also required adequately described: History of CHF: 2.0% excessive glycosuria, or Yes History of CVA: 6.5% overt hypoglycemia Current smoker: 15% ACCORD 2008;3 RCT Inclusion -

Effects of Basal Insulin Analog and Metformin on Glycaemia Control and Weight As Risk &Factors for Endothelial Dysfunction
EFFECTS OF BASAL INSULIN ANALOG AND METFORMIN ON GLYCAEMIA CONTROL AND WEIGHT AS RISK &FACTORS FOR ENDOTHELIAL DYSFUNCTION Belma Aščić – Buturović¹*, Mirsad Kacila² ¹ Clinic of Endocrinology, Diabetes Mellitus and Metabolic Diseases, University of Sarajevo Clinics Centre, Bolnička , Sarajevo, Bosnia and Herzegovina ² Center for Heart Diseases, University of Sarajevo Clinics Centre, Bolnička , Sarajevo, Bosnia and Herzegovina * Corresponding author Abstract Obese patients with type diabetes and impaired glucose tolerance are at increased risk of develop- ment of cardiovascular diseases. Endothelial dysfunction may be a reason for development of ath- erosclerosis and cardiovascular diseases. Lifestyle modifi cation, increased physical activity, weight reduction, energy restricted diet and good glycaemia control can be useful for the endothelial func- tion improvement and may decrease the risk of cardiovascular diseases. Th e aim of this study was to evaluate the eff ects of basal insulin analog and metformin on glycaemia control and weight as risk factors of endothelial dysfunction. Total of patients ( male and female) with type dia- betes were studied. Th e patients were monitored over six months period. Glycated hemoglobin (HbAc), fasting plasma glucose (FPG), postprandial plasma glucose (PPG), and body mass index (BMI) were observed. Mean age of the subjects was , ± , years. Mean diabetes duration was , ± , years. At the end of the study mean body mass index decreased from , ± , kg/m to , ±, kg/m. In this study we included diabetic patients with fasting glycaemia over mmol/ dm, postmeal glycaemia over , mmol/dm and glycated hemoglobin over . Prior to the study, the patients were treated with premix insulin divided in two daily doses and metformin after the lunch, which did not result in suffi cient regulation of glycaemia. -
Byetta (Injection Pen) Usage & Safety Guide
Byetta (Injection Pen) Usage & Safety Guide - Drugs.com 04/07/2015 Tubeless Insulin Pump Tubeless, Reliable, Safe. Try The OmniPod®. Sign-Up For A Demo Kit! Browse all medications A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Advanced Search Phonetic Search Drugs A-Z Pill Identifier Interactions Checker News Health Professionals Q & A Mednotes Apps Home → Conditions → Diabetes, Type 2 → Byetta Print Share Sign In or Register Byetta Related Information Availability Pregnancy Category Generic Name: exenatide (Byetta) (ex EN a tide) Prescription only Risk cannot be ruled out Brand Names: Byetta Prefilled Pen CSA Schedule Approval History Not a controlled drug FDA approved 2009 Overview Side Effects Dosage Interactions For Professionals More Reviews Average User Rating Prostate Cancer Stages 64 User Reviews 8.3 Rate it! See the Progression of Stages for Prostate Cancer. Get Expert Info Drug Class Incretin mimetics What is Byetta? Tubeless Byetta (exenatide) is an injectable diabetes Related Drugs medicine that helps control blood sugar levels . This medication helps Diabetes, Type 2 Insulin metformin your pancreas produce insulin more efficiently. insulin aspart Byetta is a short-acting form of exenatide. Januvia Pump Byetta is used to treat type 2 diabetes. Other glipizide Tubeless, diabetes medicines are sometimes used in glimepiride combination with Byetta if needed. Lantus Reliable, Invokana This medication guide provides information Victoza Safe. Try about the Byetta brand of exenatide. Bydureon glyburide is another brand of exenatide that is not Levemir The covered in this medication guide.