Insulin Glargine (HOE901) First Responsibilities: Understanding the Data and Ensuring Safety
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Insulin Aspart Sanofi, If It Is Coloured Or It Has Solid Pieces in It
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT Insulin aspart Sanofi 100 units/ml solution for injection in vial Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg). Insulin aspart Sanofi 100 units/ml solution for injection in vial Each vial contains 10 ml equivalent to 1,000 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Each cartridge contains 3 ml equivalent to 300 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen Each pre-filled pen contains 3 ml equivalent to 300 units insulin aspart. Each pre-filled pen delivers 1-80 units in steps of 1 unit. *produced in Escherichia coli by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection (injection). Clear, colourless, aqueous solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Insulin aspart Sanofi is indicated for the treatment of diabetes mellitus in adults, adolescents and children aged 1 year and above. 4.2 Posology and method of administration Posology The potency of insulin analogues, including insulin aspart, is expressed in units, whereas the potency of human insulin is expressed in international units. -

A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D
Drugs 2005; 65 (3): 325-340 REVIEW ARTICLE 0012-6667/05/0003-0325/$39.95/0 2005 Adis Data Information BV. All rights reserved. A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D. Mooradian Division of Endocrinology, Department of Internal Medicine, Diabetes, and Metabolism, St Louis University School of Medicine, St Louis, Missouri, USA Contents Abstract ....................................................................................325 1. Physiology of Insulin Secretion .............................................................326 2. Conventional Insulin Preparations ..........................................................327 3. Insulin Analogues ........................................................................328 3.1 Rapid-Acting Insulin Analogues .......................................................328 3.1.1 Insulin Lispro ...................................................................328 3.1.2 Insulin Aspart ..................................................................329 3.1.3 Insulin Glulisine .................................................................329 3.1.4 Clinical Utility of Rapid-Acting Insulin Analogues ...................................330 3.2 Premixed Insulins and Insulin Analogues ................................................331 3.3 Basal Insulin Analogues ...............................................................331 3.3.1 Insulin Glargine ................................................................331 -

Effects of Exenatide on Cardiac Function, Perfusion, and Energetics
Chen et al. Cardiovasc Diabetol (2017) 16:67 DOI 10.1186/s12933-017-0549-z Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine Weena J. Y. Chen1*, Michaela Diamant1^, Karin de Boer2, Hendrik J. Harms3, Lourens F. H. J. Robbers2, Albert C. van Rossum2, Mark H. H. Kramer1, Adriaan A. Lammertsma3 and Paul Knaapen2 Abstract Background: Multiple bloodglucose-lowering agents have been linked to cardiovascular events. Preliminary studies showed improvement in left ventricular (LV) function during glucagon-like peptide-1 receptor agonist administration. Underlying mechanisms, however, are unclear. The purpose of this study was to investigate myocardial perfusion and oxidative metabolism in type 2 diabetic (T2DM) patients with LV systolic dysfunction as compared to healthy controls. Furthermore, efects of 26-weeks of exenatide versus insulin glargine administration on cardiac function, perfusion and oxidative metabolism in T2DM patients with LV dysfunction were explored. Methods and results: Twenty-six T2DM patients with LV systolic dysfunction (cardiac magnetic resonance (CMR) derived LV ejection fraction (LVEF) of 47 13%) and 10 controls (LVEF of 59 4%, P < 0.01 as compared to patients) were analyzed. Both myocardial perfusion± during adenosine-induced hyperemia± (P < 0.01), and coronary fow reserve 15 (P < 0.01), measured by [ O]H2O positron emission tomography (PET), were impaired in T2DM patients as compared to healthy controls. Myocardial oxygen consumption and myocardial efciency, measured using [11C]acetate PET and CMR derived stroke volume, were not diferent between the groups. -

OZEMPIC (Semaglutide) Injection, for Subcutaneous Use Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙CONTRAINDICATIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ These highlights do not include all the information needed to use Personal or family history of medullary thyroid carcinoma or in patients OZEMPIC® safely and effectively. See full prescribing information with Multiple Endocrine Neoplasia syndrome type 2 (4). for OZEMPIC. Known hypersensitivity to OZEMPIC or any of the product components (4). OZEMPIC (semaglutide) injection, for subcutaneous use Initial U.S. Approval: 2017 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙WARNINGS AND PRECAUTIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ Pancreatitis: Has been reported in clinical trials. Discontinue promptly if WARNING: RISK OF THYROID C-CELL TUMORS pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2). See full prescribing information for complete boxed warning. Diabetic Retinopathy Complications: Has been reported in a clinical trial. Patients with a history of diabetic retinopathy should be monitored (5.3). In rodents, semaglutide causes thyroid C-cell tumors. It is Never share an OZEMPIC pen between patients, even if the needle is unknown whether OZEMPIC causes thyroid C-cell tumors, changed (5.4). including medullary thyroid carcinoma (MTC), in humans as Hypoglycemia: When OZEMPIC is used with an insulin secretagogue or the human relevance of semaglutide-induced rodent thyroid C- insulin, consider lowering the dose of the secretagogue or insulin to reduce cell tumors has not been determined (5.1, 13.1). the risk of hypoglycemia (5.5). OZEMPIC is contraindicated in patients with a personal or Acute Kidney Injury: Monitor renal function in patients with renal family history of MTC or in patients with Multiple Endocrine impairment reporting severe adverse gastrointestinal reactions (5.6). Neoplasia syndrome type 2 (MEN 2). -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -
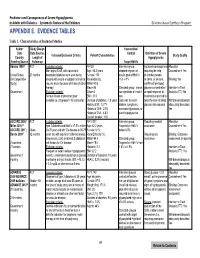
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-Based Synthesis Program APPENDIX E
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-based Synthesis Program APPENDIX E. EVIDENCE TABLES Table 1. Characteristics of Included Studies Author Study Design Intervention/ Date Data Sources Control Definition of Severe Inclusion/Exclusion Criteria Patient Characteristics Study Quality Country Length of Hypoglycemia Funding Source Follow-up Target HbA1c Abraira 199530 RCT Inclusion criteria: N=153 Intensive group: Impaired consciousness Allocation Men ages 40-69, with non-insulin Age: 60.2 years stepped regimen of requiring the help Concealment: Yes United States 27 months dependent diabetes who were being % male: 100 insulin goal of HbA1c of another person, (VA Cooperative treated with insulin or judged clinically to Race/ethnicity: =5.1+/-1% or coma, or seizure; Blinding: Yes Study) require insulin because of failure of other White=49.5 confirmed low blood therapy Black=24 Standard group: one or glucose concentration Intention-to-Treat Government Exclusion criteria: Other=3 two injections of insulin/ or rapid response to Analysis (ITT): No Serious illness or predicted poor BMI: 31.0 day treatments expected to compliance, diagnosed >15 years prior Duration of diabetes: 7.8 years Goal was to avoid raise the level of blood Withdrawals/dropouts History of MI: 13.7% diabetic symptoms, glucose also required adequately described: History of CHF: 2.0% excessive glycosuria, or Yes History of CVA: 6.5% overt hypoglycemia Current smoker: 15% ACCORD 2008;3 RCT Inclusion -

Insulin Aspart (Nvolog): Important Patient Information
What is most important to remember? If you have questions: Strong Internal Medicine • Insulin aspart (Novolog®) is used to lower blood sugar. It is Ask your doctor, nurse or pharmacist for important to use this medicine as more information about insulin aspart directed by your doctor (Novolog®) • Do not start any new medicines, over-the-counter drugs or herbal remedies without talking to your doctor • Tell all doctors, dentists and pharmacists that you are using insulin aspart (Novolog®) • insulin aspart (Novolog®) can cause low blood sugar. Always Strong Internal Medicine keep a source of sugar handy for 601 Elmwood Avenue times when your blood sugar gets Ambulatory Care Facility, 5th Floor too low Rochester, NY 14642 Phone: (585) 275 -7424 Insulin Aspart • Do not use your insulin if it (Novolog®): Visit our website at: becomes cloudy or has particles www.urmc.rochester.edu/medicine/ - Important Patient Information in it general-medicine/patientcare/ • Throw away all opened insulin after 28 days, even if it is not used up What does insulin aspart (Novolog®) do? Are there any interactions with other drugs that I need What are some things that I need to be aware of when to worry about? taking insulin aspart (Novolog®)? • It is used to lower blood sugar in patient with high blood sugar (diabetes) • There are many drug interactions that may increase • Tell your doctor or pharmacist if you have an allergy to How should insulin aspart (Novolog®) be used? your risk of side effects insulin, or any other drugs, foods, or substances • Use this -

Safety of Insulin Lispro and a Biosimilar Insulin Lispro When
DSTXXX10.1177/1932296817753644Journal of Diabetes Science and TechnologyThrasher et al 753644research-article2018 Original Article Journal of Diabetes Science and Technology 2018, Vol. 12(3) 680 –686 Safety of Insulin Lispro and a Biosimilar © 2018 Diabetes Technology Society Insulin Lispro When Administered Reprints and permissions: sagepub.com/journalsPermissions.nav Through an Insulin Pump DOI:https://doi.org/10.1177/1932296817753644 10.1177/1932296817753644 journals.sagepub.com/home/dst James Thrasher, MD1, Howard Surks, MD2, Irene Nowotny, PhD3, Suzanne Pierre, MSc4, Baerbel Rotthaeuser, PhD3, Karin Wernicke-Panten, MD3, and Satish Garg, MD5 Abstract Background: SAR342434 (U100; SAR-Lis; insulin lispro) is a biosimilar/follow-on to insulin lispro (U100; Ly-Lis). Similar pharmacokinetics/pharmacodynamics between the two products has been demonstrated in a hyperinsulinemic euglycemic clamp study. The current study evaluated the safety of SAR-Lis and Ly-Lis when administered by continuous subcutaneous insulin infusion (CSII; insulin pumps). Methods: This was a randomized, open-label, 2 × 4-week, two-arm crossover study in 27 patients with type 1 diabetes mellitus (NCT02603510). The main outcome was the incidence of infusion set occlusions (ISOs), defined as failure to correct hyperglycemia (plasma glucose ≥≥ 300 mg/dl) by 50 mg/dl within 60 minutes by insulin bolus via the pump. Secondary outcomes included intervals between infusion set changes, treatment-emergent adverse events (TEAEs) including infusion site, hypersensitivity reactions and hypoglycemic events, and safety. Results: The number of patients reporting at least one ISO was small: 6/25 patients on SAR-Lis reported 14 ISOs and 4/27 on Ly-Lis reported nine ISOs. The estimated difference in ISO risk for SAR-Lis versus Ly-Lis was 7.9% (95% CI, –1.90 to 17.73). -

Insulin Products and the Cost of Diabetes Treatment
November 19, 2018 Insulin Products and the Cost of Diabetes Treatment Insulin is a hormone that regulates the storage and use of would involve a consistent insulin level between meals sugar (glucose) by cells in the body. When the pancreas combined with a mealtime level of insulin that has a rapid does not make enough insulin (type 1 diabetes) or it cannot onset and duration of action to match the glucose peak that be used effectively (type 2 diabetes), sugar builds up in the occurs after a meal. The original insulin, also called regular blood. This may lead to serious complications, such as heart insulin, is a short-acting type of product with a duration of disease, stroke, blindness, kidney failure, amputation of action of about 8 hours, making it less suitable for toes, feet, or limbs. Prior to the discovery of insulin providing 24-hour coverage. treatment, type 1 diabetics usually died from this disease. In the late 1930s through the 1950s, regular insulin was There were 23.1 million diagnosed cases of diabetes in the altered by adding substances (protamine and zinc) to gain United States in 2015 according to the Centers for Disease longer action; these are called intermediate-acting insulins. Control and Prevention (CDC). Adding an estimated 7.2 One such advance (neutral protamine Hagedorn, or NPH) million undiagnosed cases brings the total to 30.3 million was patented in 1946 and is still in use today. It allowed for (9.4% of U.S. population). People with type 1 diabetes, the combination of two types of insulin in premixed vials about 5% of U.S. -

Complaint for Declaratory 19 and Injunctive Relief Biotechnology Innovation 20 Organization
Case 2:17-cv-02315 Document 1 Filed 09/01/17 Page 1 of 44 1 Pat Lundvall Nevada Bar No. 3761 2 MCDONALD CARANO LLP 2300 West Sahara Avenue, Suite 1200 3 Las Vegas, NV 89102 Telephone: (702) 873-4100 4 [email protected] 5 Robert N. Weiner Pending Admission Pro Hac Vice 6 Jeffrey L. Handwerker Pending Admission Pro Hac Vice 7 R. Stanton Jones Pending Admission Pro Hac Vice 8 ARNOLD & PORTER KAYE SCHOLER LLP 601 Massachusetts Avenue, NW 9 Washington, DC 20001 Telephone: (202) 942-5000 10 [email protected] [email protected] 11 [email protected] 12 Attorneys for Plaintiffs Pharmaceutical 13 Research and Manufacturers of America and Biotechnology Innovation Organization 14 15 UNITED STATES DISTRICT COURT 16 DISTRICT OF NEVADA 17 PHARMACEUTICAL RESEARCH AND Case No.: 18 MANUFACTURERS OF AMERICA, and COMPLAINT FOR DECLARATORY 19 AND INJUNCTIVE RELIEF BIOTECHNOLOGY INNOVATION 20 ORGANIZATION, 21 Plaintiffs, 22 vs. 23 BRIAN SANDOVAL, in his official capacity 24 as Governor of the State of Nevada, and 25 RICHARD WHITLEY, in his official capacity as Director of the Nevada Department for 26 Health and Human Services, 27 Defendants. 28 Case 2:17-cv-02315 Document 1 Filed 09/01/17 Page 2 of 44 1 Plaintiffs Pharmaceutical Research and Manufacturers of America (“PhRMA”) and 2 Biotechnology Innovation Organization (“BIO”) (together, “Plaintiffs”), on behalf of themselves 3 and their members, for their Complaint against Brian Sandoval, in his official capacity as Governor 4 of the State of Nevada (the “State”), and Richard Whitley, in his official capacity as Director of the 5 Nevada Department of Health and Human Services (together, “Defendants”), allege as follows: 6 INTRODUCTION 7 1. -

Inpatient Care | Lantus (Insulin Glargine Injection) 100 Units/Ml
For noncritically ill hospitalized patients with diabetes Lantus® as part of a basal-prandial dosing regimen ARA basal-prandialBBIT 2 BASAL-PRA dosing NDoptionIAL forDOS nonintensiveING care inpatients with type 2 diabetes from the RABBIT 2 Study1,a A basal-prandial dosing option for inpatients with type 2 diabetes from the RABBIT 2 Study 31 Calculate total daily dose based Total daily dose on BG and weight at the time of • For BG 140-200 mg/dL, use 0.4 Units/kg admission • For BG 201-400 mg/dL, use 0.5 Units/kg Dose administration Divide the calculated dose into basal and prandial components • Administer 50% of daily dose as basal insulin • Administer the other 50% as rapid-acting prandial 50:50 insulin divided into 3 mealtime injections basal prandial Dose administration Monitor BG, add supplemental • If fasting or mean BG during the day >140 mg/dL, increase basal insulin dose by 20% rapid-acting insulin, and adjust doses as needed • If fasting and premeal BG >140 mg/dL, add supplemental rapid-acting insulin • If BG <70 mg/dL, reduce basal insulin dose by 20% • Hold prandial insulin doses in patients not eating RABBIT 2 was a multicenter, prospective, open-label, randomized study (N=130) to compare the efficacy of a basal-prandial regimen of insulin glargine + insulin glulisine with SSI monotherapy (regular human insulin) in insulin-naive nonsurgical patients aged 18 to 80 years with type 2 diabetes. Patients in the basal-prandial group received glargine once daily and glulisine before meals. SSI was given 4 times per Aday basal-prandialfor BG >140 mg/dL.