HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GLP-1 Receptor Agonists
Cognitive Vitality Reports® are reports written by neuroscientists at the Alzheimer’s Drug Discovery Foundation (ADDF). These scientific reports include analysis of drugs, drugs-in- development, drug targets, supplements, nutraceuticals, food/drink, non-pharmacologic interventions, and risk factors. Neuroscientists evaluate the potential benefit (or harm) for brain health, as well as for age-related health concerns that can affect brain health (e.g., cardiovascular diseases, cancers, diabetes/metabolic syndrome). In addition, these reports include evaluation of safety data, from clinical trials if available, and from preclinical models. GLP-1 Receptor Agonists Evidence Summary GLP-1 agonists are beneficial for patients with type 2 diabetes and obesity. Some evidence suggests benefits for Alzheimer’s disease. It is unclear whether it is beneficial for individuals without underlying metabolic disease. Semaglutide seems to be most effective for metabolic dysfunction, though liraglutide has more preclinical data for Alzheimer’s disease. Neuroprotective Benefit: Evidence from many preclinical studies and a pilot biomarker study suggest some neuroprotective benefits with GLP-1 agonists. However, whether they may be beneficial for everyone or only a subset of individuals (e.g. diabetics) is unclear. Aging and related health concerns: GLP-1 agonists are beneficial for treating diabetes and cardiovascular complications relating to diabetes. It is not clear whether they have beneficial effects in otherwise healthy individuals. Safety: GLP-1 agonists are generally safe for most people with minor side effects. However, long-term side effects are not known. 1 Availability: Available Dose: Varies - see Chemical formula: C172H265N43O51 (Liraglutide) as a prescription chart at the end of MW: 3751.262 g/mol medicine. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Insulin Aspart Sanofi, If It Is Coloured Or It Has Solid Pieces in It
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT Insulin aspart Sanofi 100 units/ml solution for injection in vial Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg). Insulin aspart Sanofi 100 units/ml solution for injection in vial Each vial contains 10 ml equivalent to 1,000 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in cartridge Each cartridge contains 3 ml equivalent to 300 units insulin aspart. Insulin aspart Sanofi 100 units/ml solution for injection in pre-filled pen Each pre-filled pen contains 3 ml equivalent to 300 units insulin aspart. Each pre-filled pen delivers 1-80 units in steps of 1 unit. *produced in Escherichia coli by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection (injection). Clear, colourless, aqueous solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Insulin aspart Sanofi is indicated for the treatment of diabetes mellitus in adults, adolescents and children aged 1 year and above. 4.2 Posology and method of administration Posology The potency of insulin analogues, including insulin aspart, is expressed in units, whereas the potency of human insulin is expressed in international units. -

Combining a Glucagon-Like Peptide-1 Receptor Agonist with Basal Insulin: the Why and How
Combining a Glucagon-like Peptide-1 Receptor Agonist with Basal Insulin: The Why and How Case Study Mary is a 61 year-old female diagnosed with type 2 diabetes mellitus (T2DM) 8 years ago. She was initially managed with the combination of lifestyle modification and metformin. Since that time she was treated with a sulfonylurea, but it was discontinued due to symptomatic hypoglycemia. She was also treated with pioglitazone, but significant fluid retention led to it discontinuation. A year-and-a- half ago, basal insulin was added to her lifestyle and metformin management. She now administers 52 units (0.62 units/kg) once daily at bedtime. Since starting basal insulin, she has experienced 3 episodes of mild hypoglycemia. Since her diagnosis, Mary’s HbA1c has never been <7.0%; her current HbA1c is 7.9%. Over the past month, her fasting plasma glucose (FPG) has ranged from 103 mg/dL to 136 mg/dL and her postprandial glucose (PPG) from 164 mg/dL to 213 mg/dL. She has gained 2.6 kg since starting basal insulin and her body mass index is now 31 kg/m2. Her blood pressure is 134/82 mmHg. She experiences occasional tingling in her feet. Eye examination reveals grade 1 retinopathy. Current medications are: metformin 1000mg twice daily, basal insulin 52 units once daily at bedtime, and hydrochlorothiazide 25 mg once daily. Her family physician notes that Mary’s FPG is reasonably well-controlled, yet her HbA1c and PPG remain elevated. He is also concerned about her episodes of hypoglycemia and weight gain and the evidence indicating microvascular damage. -

A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D
Drugs 2005; 65 (3): 325-340 REVIEW ARTICLE 0012-6667/05/0003-0325/$39.95/0 2005 Adis Data Information BV. All rights reserved. A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D. Mooradian Division of Endocrinology, Department of Internal Medicine, Diabetes, and Metabolism, St Louis University School of Medicine, St Louis, Missouri, USA Contents Abstract ....................................................................................325 1. Physiology of Insulin Secretion .............................................................326 2. Conventional Insulin Preparations ..........................................................327 3. Insulin Analogues ........................................................................328 3.1 Rapid-Acting Insulin Analogues .......................................................328 3.1.1 Insulin Lispro ...................................................................328 3.1.2 Insulin Aspart ..................................................................329 3.1.3 Insulin Glulisine .................................................................329 3.1.4 Clinical Utility of Rapid-Acting Insulin Analogues ...................................330 3.2 Premixed Insulins and Insulin Analogues ................................................331 3.3 Basal Insulin Analogues ...............................................................331 3.3.1 Insulin Glargine ................................................................331 -

Performance of the Insulin-Only Ilet Bionic Pancreas and The
e118 Diabetes Care Volume 44, June 2021 Performance of the Insulin-Only Luz E. Castellanos,1 Courtney A. Balliro,1 Jordan S. Sherwood,1 Rabab Jafri,1 iLet Bionic Pancreas and the Mallory A. Hillard,1 Evelyn Greaux,1 Rajendranath Selagamsetty,2 Hui Zheng,3 Bihormonal iLet Using Firas H. El-Khatib,2 Edward R. Damiano,2,4 and Dasiglucagon in Adults With Type Steven J. Russell1 1 Diabetes in a Home-Use Setting Diabetes Care 2021;44:e118–e120 | https://doi.org/10.2337/dc20-1086 Reductions in blood glucose levels in with insulin lispro (Eli Lilly) or aspart (Table 1). The mean CGM glucose and people with diabetes are often achieved (Novo Nordisk), the bihormonal iLet for time in range (70–180 mg/dL) were 149 at the expense of increased hypoglyce- 7dayswithdasiglucagon(4mg/mL) ±13mg/dLand72±8%,respectively,in mia. A novel approach is to automati- and insulin lispro or aspart, or both, us- the insulin-only period, and 139 ± 11 cally deliver microdose glucagon when ing the same glucose target (110 mg/ mg/dL and 79 ± 9%, respectively, in the automation of insulin delivery alone is dL), in random order. There were no re- bihormonal period. The mean daily car- not sufficient to prevent hypoglycemia. strictions on diet or exercise. The prima- bohydrates consumed to prevent or The approach requires a bihormonal de- ry outcomes were prespecified iLet treat hypoglycemia were 16 ± 13 g and vice and a stable form of glucagon or operational thresholds. The key second- 18 ± 21 g in the insulin-only and bihor- glucagon analog. -

TRULICITY, INN-Dulaglutide
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Trulicity 0.75 mg solution for injection in pre-filled pen Trulicity 1.5 mg solution for injection in pre-filled pen Trulicity 3 mg solution for injection in pre-filled pen Trulicity 4.5 mg solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Trulicity 0.75 mg solution for injection in pre-filled pen Each pre-filled pen contains 0.75 mg of dulaglutide* in 0.5 ml solution. Trulicity 1.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 1.5 mg of dulaglutide* in 0.5 ml solution. Trulicity 3 mg solution for injection in pre-filled pen Each pre-filled pen contains 3 mg of dulaglutide* in 0.5 ml solution. Trulicity 4.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 4.5 mg of dulaglutide* in 0.5 ml solution. *produced in CHO cells by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Type 2 Diabetes Mellitus Trulicity is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. -

Effects of Exenatide on Cardiac Function, Perfusion, and Energetics
Chen et al. Cardiovasc Diabetol (2017) 16:67 DOI 10.1186/s12933-017-0549-z Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine Weena J. Y. Chen1*, Michaela Diamant1^, Karin de Boer2, Hendrik J. Harms3, Lourens F. H. J. Robbers2, Albert C. van Rossum2, Mark H. H. Kramer1, Adriaan A. Lammertsma3 and Paul Knaapen2 Abstract Background: Multiple bloodglucose-lowering agents have been linked to cardiovascular events. Preliminary studies showed improvement in left ventricular (LV) function during glucagon-like peptide-1 receptor agonist administration. Underlying mechanisms, however, are unclear. The purpose of this study was to investigate myocardial perfusion and oxidative metabolism in type 2 diabetic (T2DM) patients with LV systolic dysfunction as compared to healthy controls. Furthermore, efects of 26-weeks of exenatide versus insulin glargine administration on cardiac function, perfusion and oxidative metabolism in T2DM patients with LV dysfunction were explored. Methods and results: Twenty-six T2DM patients with LV systolic dysfunction (cardiac magnetic resonance (CMR) derived LV ejection fraction (LVEF) of 47 13%) and 10 controls (LVEF of 59 4%, P < 0.01 as compared to patients) were analyzed. Both myocardial perfusion± during adenosine-induced hyperemia± (P < 0.01), and coronary fow reserve 15 (P < 0.01), measured by [ O]H2O positron emission tomography (PET), were impaired in T2DM patients as compared to healthy controls. Myocardial oxygen consumption and myocardial efciency, measured using [11C]acetate PET and CMR derived stroke volume, were not diferent between the groups. -

OZEMPIC (Semaglutide) Injection, for Subcutaneous Use Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙CONTRAINDICATIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ These highlights do not include all the information needed to use Personal or family history of medullary thyroid carcinoma or in patients OZEMPIC® safely and effectively. See full prescribing information with Multiple Endocrine Neoplasia syndrome type 2 (4). for OZEMPIC. Known hypersensitivity to OZEMPIC or any of the product components (4). OZEMPIC (semaglutide) injection, for subcutaneous use Initial U.S. Approval: 2017 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙WARNINGS AND PRECAUTIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ Pancreatitis: Has been reported in clinical trials. Discontinue promptly if WARNING: RISK OF THYROID C-CELL TUMORS pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2). See full prescribing information for complete boxed warning. Diabetic Retinopathy Complications: Has been reported in a clinical trial. Patients with a history of diabetic retinopathy should be monitored (5.3). In rodents, semaglutide causes thyroid C-cell tumors. It is Never share an OZEMPIC pen between patients, even if the needle is unknown whether OZEMPIC causes thyroid C-cell tumors, changed (5.4). including medullary thyroid carcinoma (MTC), in humans as Hypoglycemia: When OZEMPIC is used with an insulin secretagogue or the human relevance of semaglutide-induced rodent thyroid C- insulin, consider lowering the dose of the secretagogue or insulin to reduce cell tumors has not been determined (5.1, 13.1). the risk of hypoglycemia (5.5). OZEMPIC is contraindicated in patients with a personal or Acute Kidney Injury: Monitor renal function in patients with renal family history of MTC or in patients with Multiple Endocrine impairment reporting severe adverse gastrointestinal reactions (5.6). Neoplasia syndrome type 2 (MEN 2). -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -
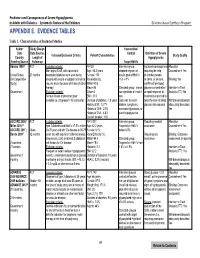
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-Based Synthesis Program APPENDIX E
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-based Synthesis Program APPENDIX E. EVIDENCE TABLES Table 1. Characteristics of Included Studies Author Study Design Intervention/ Date Data Sources Control Definition of Severe Inclusion/Exclusion Criteria Patient Characteristics Study Quality Country Length of Hypoglycemia Funding Source Follow-up Target HbA1c Abraira 199530 RCT Inclusion criteria: N=153 Intensive group: Impaired consciousness Allocation Men ages 40-69, with non-insulin Age: 60.2 years stepped regimen of requiring the help Concealment: Yes United States 27 months dependent diabetes who were being % male: 100 insulin goal of HbA1c of another person, (VA Cooperative treated with insulin or judged clinically to Race/ethnicity: =5.1+/-1% or coma, or seizure; Blinding: Yes Study) require insulin because of failure of other White=49.5 confirmed low blood therapy Black=24 Standard group: one or glucose concentration Intention-to-Treat Government Exclusion criteria: Other=3 two injections of insulin/ or rapid response to Analysis (ITT): No Serious illness or predicted poor BMI: 31.0 day treatments expected to compliance, diagnosed >15 years prior Duration of diabetes: 7.8 years Goal was to avoid raise the level of blood Withdrawals/dropouts History of MI: 13.7% diabetic symptoms, glucose also required adequately described: History of CHF: 2.0% excessive glycosuria, or Yes History of CVA: 6.5% overt hypoglycemia Current smoker: 15% ACCORD 2008;3 RCT Inclusion -

125469Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125469Orig1s000 SUMMARY REVIEW Division Director Review 1. Introduction On September 18, 2013 Eli Lilly and Company submitted a Biologics License Application (BLA) for Trulicity under section 351 of the Public Health Service Act. The applicant is seeking to indicate Trulicity as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Trulicity is a solution for injection containing either 0.75 or 1.5 mg of dulaglutide [i.e., a glucagon-like peptide 1 (GLP-1) receptor agonist]. Trulicity is to be administered by subcutaneous injection at once weekly intervals. Once approved, Trulicity will be the fifth GLP-1 agonist indicated for use in the management of patients with type 2 diabetes mellitus in the United States. This document serves as the division director’s memorandum for the application. 2. Background The drug substance in Trulicity is dulaglutide. In this memorandum, I will use Trulicity and dulaglutide interchangeably. Dulaglutide is a homodimer that consists of two identical polypeptide chains linked to each other by a disulfide bond. The polypeptide chain is a fusion protein that consists of a glucagon-like peptide 1 (GLP-1) variant, a linker, and the Fc portion of a human IgG4 variant antibody. The GLP-1 analog portion of dulaglutide is (b) (4) homologous to native human GLP-1 (7-37) (b) (4) (b) (4) Dulaglutide was demonstrated to bind and activate the GLP-1 receptor. The biological effects of endogenous GLP-1 on glucose homeostasis include augmentation of glucose stimulated insulin secretion, inhibition of glucagon release, and delay of gastric emptying.