Complaint for Declaratory 19 and Injunctive Relief Biotechnology Innovation 20 Organization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D
Drugs 2005; 65 (3): 325-340 REVIEW ARTICLE 0012-6667/05/0003-0325/$39.95/0 2005 Adis Data Information BV. All rights reserved. A Critical Appraisal of the Role of Insulin Analogues in the Management of Diabetes Mellitus Ralph Oiknine, Marla Bernbaum and Arshag D. Mooradian Division of Endocrinology, Department of Internal Medicine, Diabetes, and Metabolism, St Louis University School of Medicine, St Louis, Missouri, USA Contents Abstract ....................................................................................325 1. Physiology of Insulin Secretion .............................................................326 2. Conventional Insulin Preparations ..........................................................327 3. Insulin Analogues ........................................................................328 3.1 Rapid-Acting Insulin Analogues .......................................................328 3.1.1 Insulin Lispro ...................................................................328 3.1.2 Insulin Aspart ..................................................................329 3.1.3 Insulin Glulisine .................................................................329 3.1.4 Clinical Utility of Rapid-Acting Insulin Analogues ...................................330 3.2 Premixed Insulins and Insulin Analogues ................................................331 3.3 Basal Insulin Analogues ...............................................................331 3.3.1 Insulin Glargine ................................................................331 -

Effects of Exenatide on Cardiac Function, Perfusion, and Energetics
Chen et al. Cardiovasc Diabetol (2017) 16:67 DOI 10.1186/s12933-017-0549-z Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine Weena J. Y. Chen1*, Michaela Diamant1^, Karin de Boer2, Hendrik J. Harms3, Lourens F. H. J. Robbers2, Albert C. van Rossum2, Mark H. H. Kramer1, Adriaan A. Lammertsma3 and Paul Knaapen2 Abstract Background: Multiple bloodglucose-lowering agents have been linked to cardiovascular events. Preliminary studies showed improvement in left ventricular (LV) function during glucagon-like peptide-1 receptor agonist administration. Underlying mechanisms, however, are unclear. The purpose of this study was to investigate myocardial perfusion and oxidative metabolism in type 2 diabetic (T2DM) patients with LV systolic dysfunction as compared to healthy controls. Furthermore, efects of 26-weeks of exenatide versus insulin glargine administration on cardiac function, perfusion and oxidative metabolism in T2DM patients with LV dysfunction were explored. Methods and results: Twenty-six T2DM patients with LV systolic dysfunction (cardiac magnetic resonance (CMR) derived LV ejection fraction (LVEF) of 47 13%) and 10 controls (LVEF of 59 4%, P < 0.01 as compared to patients) were analyzed. Both myocardial perfusion± during adenosine-induced hyperemia± (P < 0.01), and coronary fow reserve 15 (P < 0.01), measured by [ O]H2O positron emission tomography (PET), were impaired in T2DM patients as compared to healthy controls. Myocardial oxygen consumption and myocardial efciency, measured using [11C]acetate PET and CMR derived stroke volume, were not diferent between the groups. -

OZEMPIC (Semaglutide) Injection, for Subcutaneous Use Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙CONTRAINDICATIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ These highlights do not include all the information needed to use Personal or family history of medullary thyroid carcinoma or in patients OZEMPIC® safely and effectively. See full prescribing information with Multiple Endocrine Neoplasia syndrome type 2 (4). for OZEMPIC. Known hypersensitivity to OZEMPIC or any of the product components (4). OZEMPIC (semaglutide) injection, for subcutaneous use Initial U.S. Approval: 2017 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙WARNINGS AND PRECAUTIONS∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ Pancreatitis: Has been reported in clinical trials. Discontinue promptly if WARNING: RISK OF THYROID C-CELL TUMORS pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2). See full prescribing information for complete boxed warning. Diabetic Retinopathy Complications: Has been reported in a clinical trial. Patients with a history of diabetic retinopathy should be monitored (5.3). In rodents, semaglutide causes thyroid C-cell tumors. It is Never share an OZEMPIC pen between patients, even if the needle is unknown whether OZEMPIC causes thyroid C-cell tumors, changed (5.4). including medullary thyroid carcinoma (MTC), in humans as Hypoglycemia: When OZEMPIC is used with an insulin secretagogue or the human relevance of semaglutide-induced rodent thyroid C- insulin, consider lowering the dose of the secretagogue or insulin to reduce cell tumors has not been determined (5.1, 13.1). the risk of hypoglycemia (5.5). OZEMPIC is contraindicated in patients with a personal or Acute Kidney Injury: Monitor renal function in patients with renal family history of MTC or in patients with Multiple Endocrine impairment reporting severe adverse gastrointestinal reactions (5.6). Neoplasia syndrome type 2 (MEN 2). -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -
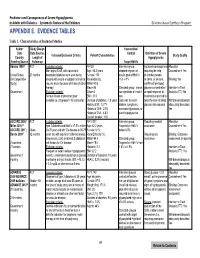
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-Based Synthesis Program APPENDIX E
Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – Systematic Review of the Evidence Evidence-based Synthesis Program APPENDIX E. EVIDENCE TABLES Table 1. Characteristics of Included Studies Author Study Design Intervention/ Date Data Sources Control Definition of Severe Inclusion/Exclusion Criteria Patient Characteristics Study Quality Country Length of Hypoglycemia Funding Source Follow-up Target HbA1c Abraira 199530 RCT Inclusion criteria: N=153 Intensive group: Impaired consciousness Allocation Men ages 40-69, with non-insulin Age: 60.2 years stepped regimen of requiring the help Concealment: Yes United States 27 months dependent diabetes who were being % male: 100 insulin goal of HbA1c of another person, (VA Cooperative treated with insulin or judged clinically to Race/ethnicity: =5.1+/-1% or coma, or seizure; Blinding: Yes Study) require insulin because of failure of other White=49.5 confirmed low blood therapy Black=24 Standard group: one or glucose concentration Intention-to-Treat Government Exclusion criteria: Other=3 two injections of insulin/ or rapid response to Analysis (ITT): No Serious illness or predicted poor BMI: 31.0 day treatments expected to compliance, diagnosed >15 years prior Duration of diabetes: 7.8 years Goal was to avoid raise the level of blood Withdrawals/dropouts History of MI: 13.7% diabetic symptoms, glucose also required adequately described: History of CHF: 2.0% excessive glycosuria, or Yes History of CVA: 6.5% overt hypoglycemia Current smoker: 15% ACCORD 2008;3 RCT Inclusion -

Insulin Glargine (HOE901) First Responsibilities: Understanding the Data and Ensuring Safety
EDITORIAL Insulin Glargine (HOE901) First responsibilities: understanding the data and ensuring safety n this issue, Heinemann et al. (1) and Rat- decline in activity through the duration of other journals. They leave the reader with ner et al. (2) provide the results of stud- the 30-h study. The authors conclude that a sense of promise, but without the firm Iies of insulin glargine (formerly known as insulin glargine provides a flatter metabolic conviction that insulin glargine is clearly HOE901), the most recent addition to the profile than NPH insulin. Theoretically, this superior to other long-acting forms of growing family of insulin analogs and for- could prove to be advantageous in accom- insulin. The pharmacodynamic study sug- mulations. The authors report insulin modating the basal insulin requirements of gests that the peakless profile of action glargine’s pharmacodynamic characteristics patients with diabetes. should provide an advantage that can be (1) and data concerning its safety and effi- Ratner et al. (2) report initial results for used to produce clinically meaningful cacy when administered to subjects with the U.S. Study Group of Insulin Glargine in improvements in glycemic control. The type 1 diabetes who were treated with reg- Type 1 Diabetes. In their article, they clinical trial’s demonstration of a reduction ular insulin (2). Insulin glargine is produced describe the results of a large randomized in hypoglycemia and in fasting plasma glu- by recombinant DNA technology with 2 prospective 28-week trial of insulin cose levels appears to provide an opportu- modifications of the native human insulin glargine versus NPH insulin. -

Insulin Glargine
Pharmacy Benefit Coverage Criteria Effective Date ............................................ 3/1/2021 Next Review Date… ..................................... 3/1/2022 Coverage Policy Number ................................ P0023 Insulin glargine Table of Contents Related Coverage Resources Medical Necessity Criteria ................................... 1 Quantity Limitations FDA Summary ..................................................... 2 Background .......................................................... 2 References .......................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s -

Fmcsa.Dot.Gov/Rules- Regulations/Administration/Fmcsr/Fmcsrguide.Asp?Section Type=A
Evidence Report Evidence Report 2010 Update: Diabetes and Commercial Motor Vehicle Driver Safety Federal Motor Carrier Safety Administration May 27, 2011 MANILA Consulting Group, Inc. 1420 Beverly Road, Suite 220 McLean, VA 22101 FMCSA Evidence Report: 2010 Update Diabetes and Commercial Motor Vehicle Driver Safety 5/27/2011 Evidence Report 2010 Update: Diabetes and Commercial Motor Vehicle Driver Safety Prepared for: Federal Motor Carrier Safety Administration U.S. Department of Transportation Prepared by: MANILA Consulting Group, Inc. 1420 Beverly Road, Suite 220 McLean, VA 22101 Authors of the 2010 update: Michelle Bieber-Tregear, PhD Damilola Funmilayo, MPH Arit Amana, MPH Dana Connor, BA Stephen Tregear, DPhil Authors of the original report: Stephen Tregear, DPhil MANILA Consulting Group Marie Tiller, PhD ECRI Institute Financial declarations: None of the authors has any affiliations or financial involvement that conflict with the material presented in this report. Policy statement This report was prepared by MANILA Consulting Group, Inc. under Contract No. GS-10F-0177N; Order No. DTMC75-10-F-00013, entitled ―Medical Programs Research and Analysis Panels Project, with the Department of Transportation’s Federal Motor Carrier Safety Administration. 2 FMCSA Evidence Report: 2010 Update Diabetes and Commercial Motor Vehicle Driver Safety 5/27/2011 Table of Contents Executive Summary ...................................................................................................................... 7 Preface ......................................................................................................................................... -

Prandial Options to Advance Basal Insulin Glargine Therapy
1318 Diabetes Care Volume 39, August 2016 Julio Rosenstock,1 Bruno Guerci,2 Prandial Options to Advance Basal Markolf Hanefeld,3 Sandro Gentile,4 Ronnie Aronson,5 Francisco J. Tinahones,6 Insulin Glargine Therapy: Testing Christine Roy-Duval,7 Elisabeth Souhami,7 Marek Wardecki,8 Jenny Ye,9 Lixisenatide Plus Basal Insulin Riccardo Perfetti,9 and Simon Heller,10 on behalf of the GetGoal Duo-2 Trial Versus Insulin Glulisine Either as Investigators Basal-Plus or Basal-Bolus in Type 2 Diabetes: The GetGoal Duo-2 Trial Diabetes Care 2016;39:1318–1328 | DOI: 10.2337/dc16-0014 CLIN CARE/EDUCATION/NUTRITION/PSYCHOSOCIAL OBJECTIVE To provide evidence-based options on how to intensify basal insulin, we explored head-to-head prandial interventions in overweight patients with type 2 diabetes inadequately controlled on basal insulin glargine with or without 1–3 oral antidi- 1Dallas Diabetes and Endocrine Center at Medi- abetic agents (OADs). cal City, Dallas, TX 2University of Lorraine and the Department of RESEARCH DESIGN AND METHODS Diabetology, Metabolic Diseases and Nutrition, Brabois Adult Hospital, Vandœuvre-les-Nancy,` Patients were randomized to lixisenatide once daily or insulin glulisine given once France 3 or thrice daily, added to glargine, with or without metformin, if HbA1c remained GWT-TUD, Study Centre Prof. Hanefeld, Dres- ‡7to£9% (‡53 to £75 mmol/mol) after 12 weeks of glargine optimization with den Technical University, Dresden, Germany 4Department of Clinical and Experimental Med- OADs other than metformin stopped at the start -

HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------------- CONTRAINDICATIONS --------------------------------- These highlights do not include all the information needed to use • TANZEUM is contraindicated in patients with a personal or family history TANZEUM safely and effectively. See full prescribing information for of medullary thyroid carcinoma or in patients with Multiple Endocrine TANZEUM. Neoplasia syndrome type 2. (4) • TANZEUM is contraindicated in patients with a prior serious TANZEUM (albiglutide) for injection, for subcutaneous use hypersensitivity reaction to albiglutide or any of the product components. Initial U.S. Approval: 2014 (4, 5.4) WARNING: RISK OF THYROID C-CELL TUMORS ------------------------- WARNINGS AND PRECAUTIONS -------------------------- See full prescribing information for complete boxed warning. • Thyroid C-Cell Tumors: See Boxed Warning. (5.1) • Carcinogenicity of albiglutide could not be assessed in rodents, • Acute Pancreatitis: Discontinue promptly if suspected. Do not restart if but other glucagon-like peptide-1 (GLP-1) receptor agonists have confirmed. Consider other antidiabetic therapies in patients with a history caused thyroid C-cell tumors in rodents at clinically relevant of pancreatitis. (5.2) exposures. Human relevance of GLP-1 receptor-agonist-induced • Hypoglycemia: Can occur when used in combination with insulin C-cell tumors in rodents has not been determined. It is unknown secretagogues (e.g., sulfonylureas) or insulin. Consider lowering whether TANZEUM causes thyroid C-cell tumors, including sulfonylurea or insulin dosage when starting TANZEUM. (5.3) medullary thyroid carcinoma (MTC), in humans. (5.1, 13.1) • Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., • TANZEUM is contraindicated in patients with a personal or angioedema) have occurred. Discontinue TANZEUM and promptly seek family history of MTC or in patients with Multiple Endocrine medical advice. -

Soliqua 100/33 Dosing and Gradual Titration Guide
SOLIQUA 100/33 DOSING AND GRADUAL TITRATION GUIDE SOLIQUA 100/33 is a combination of a long-acting human insulin analog with a glucagon-like peptide-1 (GLP-1) receptor agonist (RA) indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use: n Has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis. n Not recommended for use in combination with any other product containing a GLP-1 receptor agonist. n Not for treatment of type 1 diabetes mellitus or diabetic ketoacidosis. n Not recommended for use in patients with gastroparesis. n Has not been studied in combination with prandial insulin. Important Safety Information Contraindications Not actual patients n During episodes of hypoglycemia. n In patients with known hypersensitivity to the active substance(s) or to any of the product components. Please see full Important Safety Information on page 4. Click here for full Prescribing Information. THE POWER TO GET TO GOAL 1 SOLIQUA 100/33 allows for gradual titration of the GLP-1 RA along with the basal insulin Titration of stand-alone GLP-1 RA (lixisenatide) Gradual titration of SOLIQUA 100/33 Maintenance 60 20 19 18 50 17 16 15 14 40 Initiation 13 12 11 GLP-1 RA Dose 30 10 GLP-1 RA (mcg) Basal insulin (Units) 9 8 20 7 6 15 5 Time Time Important Safety Information Warnings and Precautions n Anaphylaxis and Serious Hypersensitivity Reactions: In clinical trials of lixisenatide, there have been cases of anaphylaxis and other serious hypersensitivity reactions including angioedema.