University Microfilms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Assessment of Molecular Action of Direct Gating and Allosteric Modulatory Effects of Carisoprodol (Somartm) on GABA a Receptors
Graduate Theses, Dissertations, and Problem Reports 2015 Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors Manoj Kumar Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Kumar, Manoj, "Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors" (2015). Graduate Theses, Dissertations, and Problem Reports. 6022. https://researchrepository.wvu.edu/etd/6022 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. ASSESSMENT OF MOLECULAR ACTION OF DIRECT GATING AND ALLOSTERIC MODULATORY EFFECTS OF MEPROBAMATE (MILTOWN®) ON GABAA RECEPTORS Manish Kumar, MD, MS Dissertation submitted to the School of Pharmacy at West Virginia University in partial fulfillment of Requirements -

Appendices Feb 2010 Inclusion of Cobalt and 2019 Fei Prohibited
APPENDIX 2 CLASSIFICATION OF PROHIBITED SUBSTANCES 1. This classification describes the types of Prohibited Substances and places same in specific categories. The list shall be published on the Club’s Notice Board. CLASS I Drugs that have the greatest pharmacological potential to affect the racing performance of a horse and which have no accepted medical use in a racehorse. These include substances which are potent stimulants of the Central Nervous System (CNS). Examples of drugs in this Class include, but are not limited to: Amphetamines, fentanyl, etorphine, cocaine, morphine, meperidine, opiates, opium derivatives, synthetic opiods, psychoactive drugs. CLASS II Drugs that have a high pharmacological potential for affecting the racing performance of a horse. These include: (i) substances which are pharmacologically active in altering the cons (ii) substances which are not generally accepted as therapeutic agents in the racing horse; and (iii) substances, some of which may have legitimate use in equine medicine but should not be found in the racing horse, such as injectable local anaesthetics. 1 Groups of drugs in this Class include, but are not limited to: (a) Opiate partial agonists, or agonistsantagonists; (b) Non-opiate psychotropic drugs, which may have stimulant, depressant, analgesic or neuroleptic effects; (c) Miscellaneous drugs which might have a stimulant effect on the CNS; (d) Drugs with prominent CNS depressant action; (e) Antidepressant and antipsychotic drugs, with or without prominent CNS stimulatory or depressant effects; (f) Muscle blocking drugs which have direct neuromuscular blocking action; (g) Local anaesthetics which have a reasonable potential for use as nerve blocking agents (except procaine); and (h) Snake venom and other biologic substances which may be used as nerve blocking agents. -
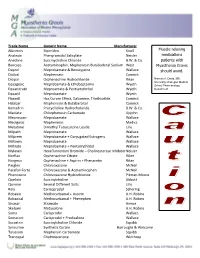
Medication Alert
Trade Name Generic Name Manufacturer Akineton Biperiden Knoll Muscle relaxing Analexin Phenyramidol Salicylate Neisler medications Anectine Succinycholine Chloride B.W. & Co. patients with Bancaps Acetaminophin, Mephenesin Butabarbital Sodium West Myasthenia Gravis Deprol Meprobamate & Benscyyzine Wallace should avoid. Dioloxl Mephensein Camrick Disipal Orphenadrine Hydrochloride Riker Norman A. David, MD, University of Oregon Medical Equagesic Meprobamate & Ethobeptazine Wyeth School Pharmacology Equanitrate Meproamate & Pentserythritol Wyeth Department Equanil Meprobamate Wyeth Flaxedil Has Curare Effect, Galiamine, Triethiodide Camrick Halabar Mephenesin & Butabarbital Camrick Kemadrin Procycllidine Hydrocholoride B.W. & Co. Maolate Chlorphenesin Carbamate Upjohn Meprospan Meprobamate Wallace Medigesic Mephenesin Medics Metubine Dimethyl Tubocurzine Loside Lilly Milpath Meprobamate Wallace Milprem Meprobamate + Conjugated Estrogens Wallace Miltown Meprobamate Wallace Miltrate Meprobamate + Pentserythritol Wallace Mylaxen Hexafluroenium Bromide – Cholinesterase Inhibitor Neisler Norflax Orphenadrine Citrate Riker Norgesic Orphenadrine + Aspirin + Phenacetin Riker Parglex Chlorzoxazone McNeil Parafon Forte Chlorzoxazone & Acetaminophen McNeil Phenoxene Chlorzoxazone Hydrochloride Pitman-Moore Quelicin Succinylcholine Abbott Quinine Several Different Salts Lilly Rela Carisoprodol Schering Robaxin Methocarbamal + Aspirin A.H. Robins Robaxisal Methocarbamal + Phenephen A.H. Robins Sinaxar Stramate Armor Skelaxin Metaxalone A.H. Robins Soma -

Carisoprodol (CAS No
National Toxicology Program Toxicity Report Series Number 56 NTP Technical Report on the Toxicity Studies of Carisoprodol (CAS No. 78-44-4) Administered by Gavage to F344/N Rats and B6C3F1 Mice August 2000 U.S. Department of Health and Human Services Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Toxicity Study Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. These studies are designed and conducted to characterize and evaluate the toxicologic potential of selected chemicals in laboratory animals (usually two species, rats and mice). -

Ethiopian Food and Drug Authority Annexs to Cosmetics Import, Export and Wholesale Control Directive No. 48/2020
Ethiopian Food and Drug Authority Annexs to Cosmetics Import, Export and Wholesale Control Directive No. 48/2020 March, 2020 Addis Ababa, Ethiopia 1 Annexes to the Cosmetics Import, Export and Wholesale Control Directive No. 48/2020 1) Annex I: Illustrative list of cosmetics by catagories 2) Annex II: List of prohibited substances 3) Annex III: List of substances which cosmetic must not contain except the restrictions laid down 4) Annex IV: List of colorants allowed in cosmetics 5) Annex V: List of preservatives allowed in cosmetics 2 Annex I ILLUSTRATIVE LIST OF COSMETICS BY CATEGORIES Creams, emulsions, lotions, gels and oils for the skin (hands, face, feet, etc.) Face masks (with the exception of chemical peeling products) Tinted bases (liquids, pastes, powders) Make-up powders, after-bath powders, hygienic powders, etc. Toilet soaps, deodorant soaps, etc. Perfumes, toilet waters and eau de cologne Bath and shower preparations (salts, foams, oils, gels, etc.) Depilatories Deodorants and anti-perspirants Hair care products hair tints and bleaches products for waving, straightening and fixing setting products o cleansing products (lotions, powders, shampoos) conditioning products (lotions, creams, oils) hairdressing products (lotions, lacquers, brilliantines) Shaving products (creams, foams, lotions, etc.) Products for making-up and removing make-up from the face and the eyes Products intended for application to the lips Products for care of the teeth and the mouth Products for nail care and make-up Products for -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Gamma Dosimetric Parameters in Some Skeletal Muscle Relaxants
Pramana – J. Phys. (2017) 89:42 © Indian Academy of Sciences DOI 10.1007/s12043-017-1440-x Gamma dosimetric parameters in some skeletal muscle relaxants H C MANJUNATHA Department of Physics, Government College for Women, Kolar 563 101, India E-mail: [email protected] MS received 13 October 2015; revised 29 April 2017; accepted 12 May 2017; published online 29 August 2017 Abstract. We have studied the attenuation of gamma radiation of energy ranging from 84 keV to 1330 keV (170Tm,22Na,137Cs, and 60Co) in some commonly used skeletal muscle relaxants such as tubocurarine chloride, gallamine triethiodide, pancuronium bromide, suxamethonium bromide and mephenesin. The mass attenuation coefficient is measured from the attenuation experiment. In the present work, we have also proposed the direct relation between mass attenuation coefficient (μ/ρ) and mass energy absorption coefficient (μen/ρ) based on the nonlinear fitting procedure. The gamma dosimetric parameters such as mass energy absorption coefficient γ (μen/ρ), effective atomic number (Zeff ), effective electron density (Nel), specific -ray constant, air kerma strength and dose rate are evaluated from the measured mass attentuation coefficient. These measured gamma dosimetric parameters are compared with the theoretical values. The measured values agree with the theoretical values. The studied gamma dosimetric values for the relaxants are useful in medical physics and radiation medicine. Keywords. Effective atomic number; electron density; CT number; relaxants. PACS Nos 29.20.−c; 29 1. Introduction round of radiotherapy treatment, muscle relaxants are injected to relieve the pain. During the second or subse- In radiotherapy, γ-rays are capable of interacting with quent cycles, some muscle relaxants still remain in the tissues for treatment of malignant neoplasias. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0