Carisoprodol (CAS No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

University Microfilms
INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You w ill find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Misperceptions About Contraceptives Persist
30 Women’s Health FAMILY P RACTICE N EWS • September 1, 2008 Misperceptions About Contraceptives Persist BY DOUG BRUNK trauterine device use; 50% thought IUD (SOGC). There may be inadequate in- Oral contraceptives are also safe in women San Diego Bureau failure was a major risk; and fewer than struction with regard to contraception in older than age 35 if they are healthy non- one-third would recommend an IUD as an physician-training programs, she said. smokers, and in women with systemic lu- C ALGARY, ALTA. — Physicians may option for nulliparous women, for post- “Not everybody does IUD insertions, sub- pus erythematosus provided they have no have a surprising number of misconcep- coital contraception, for women with fi- dermal progestin implant, or vasectomy.” antiphospholipid antibodies, vascular dis- tions about birth control, according to re- broids, or for women who had pelvic in- Other barriers to effective contraceptive ease, or nephritis. cent responses to a survey by 96 family flammatory disease within the last year use include exaggerated concerns about Another common exaggerated concern physicians in Kingston, Ont. (Can. Fam. Physician 2008;54:560-6). potential side effects. For example, women is that the use of oral and transdermal Contrary to evidence in the medical lit- “Only 41% of these physicians inserted with diabetes may use oral contraceptives patch contraceptives cause weight gain. erature, more than 60% of respondents IUDs,” Dr. Amanda Black said at the an- if they have no end-organ damage, said Dr. However, a recent Cochrane systematic re- thought pelvic inflammatory disease and nual meeting of the Society of Obstetri- Black of the division of general obstetrics view of randomized, controlled data con- ectopic pregnancy were major risks of in- cians and Gynaecologists of Canada and gynecology at the Ottawa Hospital. -

Forensic Toxicology Laboratory Office of Chief Medical Examiner City of New York
FORENSIC TOXICOLOGY LABORATORY OFFICE OF CHIEF MEDICAL EXAMINER CITY OF NEW YORK CARISOPRODOL , MEPROBAMATE and TOPIRAMATE by SOLID PHASE EXTRACTION and GAS CHROMATOGRAPHY/MASS SPECTROMETRY (Selected Ion Monitoring) PRINCIPLE Carisoprodol is a carbamate derivative first synthesized in 1959. It is primarily used as a muscle relaxant. UncontrolledMeprobamate is also a carbamate derivative used as a muscle relaxant and the primary metabolite of carisoprodol. Topiramate is a sulfamate-substituted monosaccharide used as an anticonvulsant Carisoprodol, meprobamate and topiramate are quantitated by a selected ion monitoring (SIM) method using methapyrilene as the internal standard. Carisoprodol, meprobamate and topiramate are extracted from biological specimens by solid phase extraction. Drugs are temporarily bound to a sorbent in the solid phase cartridge as the prepared sample is poured through the column. The column is washed to remove interfering compounds, followed by elution of drugs from the column. The eluate is evaporated, reconstituted and injected in the GCMS. Quantitative analysis is performed by SIM GCMS using a six point calibration curve. SAFETY The handling of all biological specimens and reagents is performed within the guidelines which are detailed in the Safety and Health manual. Copy SPECIMEN PREPARATION The procedure is routinely applied to the following biological specimens and their aliquots unless otherwise specified: Blood 0.5 mL of the undiluted specimen Urine 0.5 mL for qualitative identification Brain 0.5 mL of a 1:3 homogenate Gastric Contents 0.5 mL of a 1:10 dilution Liver 0.5 mL of a 1:5 homogenate Vitreous Humor 0.5 mL of the undiluted specimen Bile 0.5 mL of the undiluted specimen Reviewed by: Date: Page 1 of 14 T:\FINALSOP\FINAL SOP PDF\G. -

Metabolism of Drugs in Old Rats (Ii) Metabolism in Vivo and Effect of Drugs in Old Rats
METABOLISM OF DRUGS IN OLD RATS (II) METABOLISM IN VIVO AND EFFECT OF DRUGS IN OLD RATS RYUICHI KATO AND AKIRA TAKANAKA Department of Pharmacology, National Institute of Hygienic Sciences, Setagaya-ku, Tokyo Received for publication March 16, 1968 It is well known that the effects of some drugs in old animals quite differ from those in young animals. For example, Farner and Verzar (1) observed that the action of am phetamine to increase moter activity was more marked in young rats than in old rats and the action of hexobarbital to antagonized against the amphetamine action was more ef fective in old rats than in young rats. Moreover, the duration of hexobarbital anesthesia was longer in old rats than in young rats (2). Similarly, Petty and Karler (3) observed that anticonvulsive activity of acetazolamide and phenobarbital was more marked in old rats than in young rats. However, there is no study on the mechanism of such altered drug response in rela tion to the rate of drug metabolism. In a previous paper it was reported from our labo ratory that the activities in the oxidation and reduction of drugs and NADPH-linked elec tron transport system in liver microsomes of male and female rats were progressively decreased with aging (4). It is, therefore, of interest to investigate whether or not the metabolism in vivoof vari ous drugs is decreased in old rats in accordance with the decrease in the metabolic activi ties of liver microsomes observed in the in vitro experiments. Moreover, the relationship between the effects of drugs and the rate of in vivo metabolism of drugs in the old rats was studied. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Assessment of Molecular Action of Direct Gating and Allosteric Modulatory Effects of Carisoprodol (Somartm) on GABA a Receptors
Graduate Theses, Dissertations, and Problem Reports 2015 Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors Manoj Kumar Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Kumar, Manoj, "Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors" (2015). Graduate Theses, Dissertations, and Problem Reports. 6022. https://researchrepository.wvu.edu/etd/6022 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. ASSESSMENT OF MOLECULAR ACTION OF DIRECT GATING AND ALLOSTERIC MODULATORY EFFECTS OF MEPROBAMATE (MILTOWN®) ON GABAA RECEPTORS Manish Kumar, MD, MS Dissertation submitted to the School of Pharmacy at West Virginia University in partial fulfillment of Requirements -

Open PDF File, 64.59 KB, for Carisoprodol Letter
The Commonwealth of Massachusetts Executive Office of Health and Human Services Office of Medicaid 600 Washington Street Boston, MA 02111 MITT ROMNEY TIMOTHY MURPHY Secretary Governor KERRY HEALEY BETH WALDMAN Medicaid Director Lieutenant Governor September 1, 2005 Dear Prescriber: As of October 3, 2005, all products containing carisoprodol will require prior authorization when prescribed for MassHealth members. This change reflects MassHealth’s and the Drug Utilization Review (DUR) Board’s review of carisoprodol. Carisoprodol has shown limited efficacy in the relief of acute pain associated with musculoskeletal conditions, as an adjunct to rest, physical therapy, and other measures. Carisoprodol does not act directly on the skeletal muscles, but its effects are thought to be related to its sedative properties. Carisoprodol is metabolized to meprobamate, a Schedule IV anxiolytic with a known potential for abuse. According to data collected by the Drug Abuse Warning Network (DAWN), a surveillance system operated by the U.S. Department of Health and Human Services, carisoprodol had a similar abuse pattern to meprobamate. Carisoprodol abuse was often identified in combination with other drugs of abuse such as benzodiazepines, opioid analgesics, alcohol and barbiturates*. Carisoprodol abuse can lead to seizures, coma, and death. The MassHealth Drug List can be found at www.mass.gov/druglist. We would encourage you to review the site because it provides valuable information about the pharmacy benefit for MassHealth members. Specifically, the List lets you know which drugs require prior authorization in the MassHealth program and provides other useful clinical information. We also encourage you to use the MassHealth Web site to subscribe to our service that will automatically send you e-mail alerts when the MassHealth Drug List is updated. -
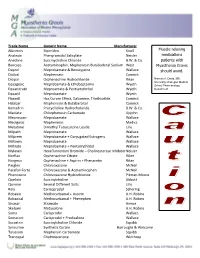
Medication Alert
Trade Name Generic Name Manufacturer Akineton Biperiden Knoll Muscle relaxing Analexin Phenyramidol Salicylate Neisler medications Anectine Succinycholine Chloride B.W. & Co. patients with Bancaps Acetaminophin, Mephenesin Butabarbital Sodium West Myasthenia Gravis Deprol Meprobamate & Benscyyzine Wallace should avoid. Dioloxl Mephensein Camrick Disipal Orphenadrine Hydrochloride Riker Norman A. David, MD, University of Oregon Medical Equagesic Meprobamate & Ethobeptazine Wyeth School Pharmacology Equanitrate Meproamate & Pentserythritol Wyeth Department Equanil Meprobamate Wyeth Flaxedil Has Curare Effect, Galiamine, Triethiodide Camrick Halabar Mephenesin & Butabarbital Camrick Kemadrin Procycllidine Hydrocholoride B.W. & Co. Maolate Chlorphenesin Carbamate Upjohn Meprospan Meprobamate Wallace Medigesic Mephenesin Medics Metubine Dimethyl Tubocurzine Loside Lilly Milpath Meprobamate Wallace Milprem Meprobamate + Conjugated Estrogens Wallace Miltown Meprobamate Wallace Miltrate Meprobamate + Pentserythritol Wallace Mylaxen Hexafluroenium Bromide – Cholinesterase Inhibitor Neisler Norflax Orphenadrine Citrate Riker Norgesic Orphenadrine + Aspirin + Phenacetin Riker Parglex Chlorzoxazone McNeil Parafon Forte Chlorzoxazone & Acetaminophen McNeil Phenoxene Chlorzoxazone Hydrochloride Pitman-Moore Quelicin Succinylcholine Abbott Quinine Several Different Salts Lilly Rela Carisoprodol Schering Robaxin Methocarbamal + Aspirin A.H. Robins Robaxisal Methocarbamal + Phenephen A.H. Robins Sinaxar Stramate Armor Skelaxin Metaxalone A.H. Robins Soma -

Clinical Pharmacology and Biopharmaceutics Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200327 CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S) CLINICAL PHARMACOLOGY REVIEW NDA: 200-327 Submission Date(s): • 30 Dec 2009 (SDN 1) • 30 Apr 2010 (SDN 14) • 04 Feb 2010 (SDN 7) • 18 Jun 2010 (SDN 19) • 23 Apr 2010 (SDN 10) • 06 Aug 2010 (SDN 31) • 29 Apr 2010 (SDN 13) • 18 Aug 2010 (SDN 34) Drug Ceftaroline Fosamil for Injection Trade Name TEFLARO™ (proposed) OCP Reviewer Aryun Kim, Pharm.D. OCP Team Leader Charles Bonapace, Pharm.D. PM Reviewer Yongheng Zhang, Ph.D. PM Team Leader Pravin Jadhav, Ph.D. OCP Division DCP4 OND division DAIOP (520) Sponsor Cerexa, Inc., Oakland, CA Relevant IND(s) IND 71,371 Submission Type; Code Original New Drug Application (New Molecular Entity), 1S Formulation; Strength(s) Sterile (b) (4) of ceftaroline fosamil and L-arginine supplied as powder in single-use, 20-cc, clear, Type I glass vials containing 600 mg or 400 mg of ceftaroline fosamil Indication For the treatment of complicated skin and skin structure infections (cSSSI) and community-acquired bacterial pneumonia (CABP) caused by designated susceptible isolates of Gram-positive and Gram-negative microorganisms Dosage and 600 mg administered every 12 hours by intravenous infusion over 1 hour Administration in patients ≥18 years of age • for 5-14 days for treatment of cSSSI • for 5-7 days for treatment of CABP 1. EXECUTIVE SUMMARY 5 1.1 Recommendations 5 1.2 Phase 4 Commitments 6 1.3 Summary of Important Clinical Pharmacology and Biopharmaceutics Findings 6 2. QUESTION-BASED REVIEW 11 2.1 General Attributes of the Drug 11 2.2 General Clinical Pharmacology 13 2.3 Intrinsic Factors 37 2.4 Extrinsic Factors 56 2.5 General Biopharmaceutics 58 2.6 Analytical Section 58 3. -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and