Long-Term Dynamics of Plant Communities After Biological
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -
Species Lists
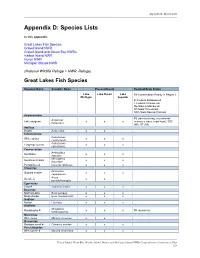
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -

Biological Activities of Trifolium Pratense: a Review
Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423) Volume 3 Issue 9 September 2019 Review Article Biological Activities of Trifolium Pratense: A Review Atiq-ur-Rehman1,2* 1University College of Pharmacy, University of the Punjab, Lahore, Pakistan 2Faculty of Pharmacy, The University of Lahore, Lahore, Pakistan *Corresponding Author: Atiq-ur-Rehman, Faculty of Pharmacy, The University of Lahore and University College of Pharmacy, University of the Punjab, Lahore Pakistan. Received: July 25, 2019; Published: August 16, 2019 Abstract Trifolium pratense is an important plant of the Legume family. It has drawn the attention of several researchers around the globe. This plant was traditionally used as forage or as soil improver is now seen as the plant containing vast therapeutic activities which include anti-oxidative, anti-cancer, neuroprotective, anti-hyperglycemic, anti-hyperlipidemic, osteoprotective and cardio protective properties. The therapeutic properties are shown in various in vivo, in vitro and ex vivo experiments. The review highlights the Tri- forium pratense basic knowledge its extraction, components and their actions, major activities possessed by plant along with their mechanisms. Trifolium plant is mainmajorly used in menopausal women to reduce the discomfort and menopausal effects such as moderate cancer causing cells. Various strategies were applied and the plant is still under study for further development in its effects. hot flushes and increase in breast density. The plant is also majorly responsible for preventing breast cancer and other apoptosis of Keywords: Trifolium Pratense; Cancer; Trifolium Introduction Family The genus Trifolium comprises of almost 240 species each re- It belongs to the family Fabeaceae leguminosae. markable for its agricultural and therapeutic effects. -

RED CLOVER This Plant May Become Weedy Or Invasive in Some Regions Or Habitats and May Displace Other Vegetation Trifolium Pratense L
Plant Guide Weediness RED CLOVER This plant may become weedy or invasive in some regions or habitats and may displace other vegetation Trifolium pratense L. if not properly managed. Consult your local NRCS Plant Symbol = TRPR2 Field Office, Cooperative Extension Service Office, or state natural resource or agriculture department Contributed by: Idaho Plant Materials regarding its status and use. Weed information is also available from the PLANTS web site at plants.usda.gov Description Trifolium pratense L., red clover, is an introduced biennial or short-lived perennial that grows as one of two types: medium (double-cut) or mammoth (single- cut). Red clover initiates growth from the plant crown. Plants have hollow, hairy stems and branches. Stem lengths of medium and mammoth types average 18 inches and 24-30 inches, respectively. Medium types have about 4 branches per stem; mammoth types have 6 branches per stem. USDA NRCS PLANTS Each leaf consists of a slender stalk bearing 3 leaflets. The taproot of red clover normally disintegrates in the second year and plants that Uses survive have developed secondary, extensively Red clover is the most widely grown of all the true branched roots. Flowers are borne in compact clovers and is the most important legume hay crop in clusters at the tips of the branches and are usually the northeastern United States. Red clover is rose-pink in color. The flower shape is similar to pea primarily used for hay, pasture, silage, and soil flowers except is more elongated and much smaller. improvement. It is a quick growing crop, easily Flower heads usually consist of up to 125 flowers. -

Conservation Plants Pocket ID Guide
About this guide.......... The purpose of this guide is to help you identify come commonly used conservation plants. Its color photos, line drawings and seed photos will help you make identifications. Also included are plant stand evaluation and recommended use charts. Keep this guide with you as long as you need it! Uses 1 2 Evaluating Stands Seeding success may not be obvious from visual observation. Use the chart below to determine whether your first-year stand is adequate. Lay a square-foot frame, or a circular frame with a 42.5-inch circumference on the ground. Count the number of seedlings within the frame, taking at least 10 counts for each 10 acres, in representative areas of the field. The table is based on pure stands; if a mixture of grass and legume is planted, reduce the numbers by the ratio of each species planted. Inadequate stands should be re-seeded. When a stand is judged to be between adequate and inadequate, it should be reevaluated after the second growing season. Warm-season grasses may need to be evaluated after the third growing season. 3 Seeds Early ID -- Seed is Key Grasses can be very difficult to identify in early growth stages. The seed may be the best identify aid. A seed retains its form and position in the ground through the seedling's early growth stages. To identify a seedling, carefully dig it up and compare it to photographs or actual seeds. 4 Plant Parts Conservation Plants The Elsberry Plant Materials Center The Elsberry Plant Materials Center is a 243-acre facility near Elsberry, Missouri. -

Comparative Analysis of Nodulation-Related Small Secreted Peptides Across Legume Species
Comparative analysis of nodulation-related small secreted peptides across legume species A DISSERTATION SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA BY Diana Trujillo IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Nevin Young November 2017 © Diana Trujillo 2017 Acknowledgments I would like to thank all those who, one way or another, have made this work possible. First and foremost, thanks go to my advisor, Nevin Young, who helped shape my vision for this project and was always available to give me support or the necessary guidance in the right direction. I would also like to thank Kevin Silverstein who played a large role during the development of my LSE pipeline, and was a helpful mentor in bioinformatic matters. Thanks to the other members of my advisory committee, Peter Morrell, Michael Sadowsky, and Robert Stupar, who provided a fresh outlook and valuable advice that helped to improve this study. I would like to thank Joseph Guhlin and Peng Zhou who were always one step away when I had Unix concerns, and Shaun Curtin and Roxanne Denny who guided me through the technical aspects of growing or transforming Medicago. I am grateful that I had a strong network of friends and colleagues to discuss biology, coding, and life. To Allison Haaning, Beth Fallon, Christina Smith, Leland Werden, Derek Nedveck, and Eli Krumholz: thanks for sharing the journey. I would like to thank my mother, María Cecilia, who instilled my love of reading, truth-seeking, and plants. To my husband, Dylan Huss, thank you for always calling to see if I already ate (including as I typed these words). -

Biology of a New Virus Isolated from Lupinus Nootkatensis Plants in Alaska
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2004 Biology of a new virus isolated from Lupinus nootkatensis plants in Alaska N. L. Robertson USDA, ARS Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Robertson, N. L., "Biology of a new virus isolated from Lupinus nootkatensis plants in Alaska" (2004). Publications from USDA-ARS / UNL Faculty. 1443. https://digitalcommons.unl.edu/usdaarsfacpub/1443 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Plant Pathology (2004) 53, 569–576 Doi: 10.1046/j.0032-0862.2004.01058.x BlackwellBiology Publishing, Ltd. of a new virus isolated from Lupinus nootkatensis plants in Alaska N. L. Robertson USDA, ARS, Arctic Plant Germplasm Introduction and Research Project, 533 E. Fireweed Avenue, Palmer, AK 99645, USA A new virus named Nootka lupine vein-clearing virus (NLVCV) was isolated from Lupinus nootkatensis plants that were confined to a relatively small area in the Talkeetna mountains of south-central Alaska. Annual surveys (2000–03) con- sistently found leaf symptoms of pronounced vein clearing and mosaic on 3- to 4-week-old plants in late June. Spherical particles ≈30 nm in diameter were isolated from these leaves. Virions contained a single-stranded RNA of ≈4·0–4·2 kb and one species of capsid protein estimated to be ≈40 kDa. -

2.4 FORAGE LEGUMES MILLETS and TIMOTHY.Pdf
2.4 FORAGE LEGUMES, MILLETS AND TIMOTHY** Alfalfa, clovers, sweet clover, crown vetch, kidney vetch, cicer milkvetch, birds-foot’s trefoil, black medick, timothy, millets (Grade Tables VIII to X) ** Note: MIXTURES Forage mixtures, lawn mixtures, and ground cover mixtures (Grade Tables XIII to XV), prerequisite accreditation for forage legumes, millets and timothy and grasses English Common Picture Grade Y/N Botanical Name Full Botanical Name & References Tables Name French Common Name Family y Anthyllis vulneraria Anthyllis vulneraria L. VIII Vetch, kidney Anthyllide vulnéaire Fabaceae y Astragalus cicer Astragalus cicer L. VIII Milk-vetch, cicer Astragale pois chiche Fabaceae Echinochloa frumentacea Link or E. esculenta (A. Brown) H. Scholz. (=E. crus-galli (L.) P. Beauv. var. frumentacae y Echinochloa frumentacea (Link) E. G. Camus & A. Camus) VIII Millet, Japanese Millet japonais Poaceae Kummerowia stipulacea (Maxim.) Makino (=Lespedeza y Kummerowia stipulacea stipulacea Maxim.) VIII Lespedeza. Korean Lespédeza, de Corée Fabaceae Kummerowia striata (Thunb.) Schindl. (=Lespedeza Lespedeza, common or y Kummerowia striata striata (Thunb.) Hook. & Arn.) VIII Kobe Lespédeza, commun ou kobe Fabaceae Lespedeza, sericea or Lespédeza, sericea ou de y Lespedeza cuneata Lespedeza cuneata (Dum.-Cours.) G. Don VIII Chinese Chine Fabaceae y Lotus corniculatus Lotus corniculatus L. X Trefoil, bird's foot Lotier corniculé Fabaceae y Medicago lupulina Medicago lupulina L. IX Medick, black Lupuline Fabaceae y Medicago sativa Medicago sativa L. VIII Alfalfa Luzerne Fabaceae Clover sweet - white Mélilot ou trèfle d'odeur - y Melilotus albus Melilotus albus Medik. VIII blossom fleurs blanches Fabaceae Clover sweet - yellow Mélilot ou trèfle d'odeur - y Melilotus officinalis Melilotus officinalis (L.) Lam. -

Nectar and Pollen Plants
NECTAR AND POLLEN PLANTS BY EVERETT OEETEL > A beekeeper must have available data on the Beekeepers are advised to record the blossoming nectar and pollen plants in the vicinity of his api- period for the nectar and pollen plants in their ary for successful honey production. Such informa- vicinity (fig. 1). Most State agricultural extension tion enables him to determine when to install services have publications available on beekeeping. package bees, divide colonies, put on supers, use These publications usually contain a list of the swarm-control measures, remove honey, requeen, important nectar and pollen plants. Unknown prepare colonies for winter, and locate profitable plants can be sent to the botany department of the apiary sites. State university for identification. Vansell {1931) listed 150 species of nectar and pollen plants in California, but only six are princi- Beekeeping Locations pal sources for commercial honey production. He listed about 90 species of nectar and pollen plants Beekeepers, especially commercial operators, in Utah but noted that the main sources of com- have learned that the nectar- and pollen-producing mercial honey are alfalfa and sweetclover (1949). plants may change considerably over the years. Wilson et al. (1958) observed honey bees visiting Variations may be caused by droughts, changes in the blossoms or extrafloral nectaries of 110 species agricultural crops and practices, irrigation projects, of plants in Colorado, of which the most important and subdivision development. Changes have been honey sources were alfalfa, yellow sweetclover, and particularly rapid since World War II and are dandelion (fig. 1). likely to continue. Acreages planted to buckwheat, alsike clover, and cotton have decreased, whereas those with alfalfa hay, mustard, safflower, and soybeans have increased. -

Cytoplasmic Inheritance of Plastids in Impatiens Sultanii Hook, F., Petunia
CYTOPLASMIC INHERITANCE OF PLASTIDS IN IMPATIENS SULTANII HOOK, P., PETUNIA VIOLACEA LINDL. AND CHOLROPHYTUM ELATUM R. BR.1 K. K. PANDEY AND GLENN W. BLAYDES Department of Botany and Plant Pathology, The Ohio State University, Columbus 10 INTRODUCTION The inheritance and mode of chlorophyll distribution in variegated plants have long attracted the attention of botanists. It is probable that most variegated varieties of plant species, domesticated and wild, are somatic, mutant individuals which appear as chimeras (Blaydes, 1953). Many of these may be propagated vegetatively and persist indefinitely through the aid of man. However, the understanding of the processes involved in the structure and inheritance of leaf variegation is still far from complete. A favorable condition for the study of chlorophyll inheritance is found in plants where one or more shoots are completely albino. Such shoots are incapable of independent existence, except when under special cultural methods, and may be found as mutants or somatic segregates on green or variegated plants (Blaydes, 1953). The most frequent occurrence of such albino mutants is found in periclinally, mericlinally and sectorially variegated plants. Since flowers on albino shoots are frequently unable to produce seed normally, mericlinal, periclinal, or sectorial chimeras are generally used for genetical experiments. As the repro- ductive cells are derived from the sub-epidermal layer of the shoot, the second layer of the shoot apex must be regularly albino if the genetic data from crosses in such plants are to be relied upon. Sectorially variegated plants in which the histogenic tissues are constantly rearranging are not reliable for genetic studies on variegation. -

Minnesota Landscape Arboretum Bloom List 6/24/2011
Minnesota Landscape Arboretum Bloom List 6/24/2011 Botanical name....................................................................................... Common Name Location(s) Achillea millefolium ...............................................................................................Yarrow Bog Area, Prairie, Prairie Display Garden Achillea spp. ...........................................................................................................Yarrow Home Demonstration Garden, Perennial Garden, Rock Garden, Snyder Building Alchemilla glaucescens................................................................................ Lady's Mantle Rock Garden Alchemilla spp. ............................................................................................ Lady's Mantle Home Demonstration Garden, Peony Collection, Sensory Garden Allium spp................................................................................................................Allium Home Demonstration Garden, Rock Garden Allium tricoccum ............................................................................................... Wild Leek Wildflower Garden Amorpha canescens ............................................................................................ Leadplant Sensory Garden Amsonia illustria....................................................................................................Bluestar Wildflower Garden Amsonia tabernaemontana.......................................................................Willow Amsonia Arboretum