The Use of Red Clover (Trifolium Pratense) in Soil Fertility-Building: a Review Patrick Mckenna⁎, Nicola Cannon, John Conway, John Dooley
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses
pathogens Review Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses Daniele Cristina Fontana 1,† , Samuel de Paula 2,*,† , Abel Galon Torres 2 , Victor Hugo Moura de Souza 2 , Sérgio Florentino Pascholati 2 , Denise Schmidt 3 and Durval Dourado Neto 1 1 Department of Plant Production, Luiz de Queiroz College of Agriculture, University of São Paulo, Piracicaba 13418900, Brazil; [email protected] (D.C.F.); [email protected] (D.D.N.) 2 Plant Pathology Department, Luiz de Queiroz College of Agriculture, University of São Paulo, Piracicaba 13418900, Brazil; [email protected] (A.G.T.); [email protected] (V.H.M.d.S.); [email protected] (S.F.P.) 3 Department of Agronomy and Environmental Science, Frederico Westphalen Campus, Federal University of Santa Maria, Frederico Westphalen 98400000, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-54-99646-9453 † These authors contributed equally to this work. Abstract: Plant diseases cause losses of approximately 16% globally. Thus, management measures must be implemented to mitigate losses and guarantee food production. In addition to traditional management measures, induced resistance and biological control have gained ground in agriculture due to their enormous potential. Endophytic fungi internally colonize plant tissues and have the potential to act as control agents, such as biological agents or elicitors in the process of induced resistance and in attenuating abiotic stresses. In this review, we list the mode of action of this group of Citation: Fontana, D.C.; de Paula, S.; microorganisms which can act in controlling plant diseases and describe several examples in which Torres, A.G.; de Souza, V.H.M.; endophytes were able to reduce the damage caused by pathogens and adverse conditions. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -
Species Lists
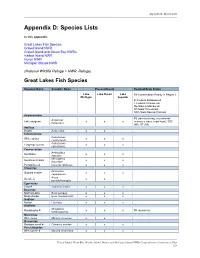
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -

Colletotrichum – Names in Current Use
Online advance Fungal Diversity Colletotrichum – names in current use Hyde, K.D.1,7*, Cai, L.2, Cannon, P.F.3, Crouch, J.A.4, Crous, P.W.5, Damm, U. 5, Goodwin, P.H.6, Chen, H.7, Johnston, P.R.8, Jones, E.B.G.9, Liu, Z.Y.10, McKenzie, E.H.C.8, Moriwaki, J.11, Noireung, P.1, Pennycook, S.R.8, Pfenning, L.H.12, Prihastuti, H.1, Sato, T.13, Shivas, R.G.14, Tan, Y.P.14, Taylor, P.W.J.15, Weir, B.S.8, Yang, Y.L.10,16 and Zhang, J.Z.17 1,School of Science, Mae Fah Luang University, Chaing Rai, Thailand 2Research & Development Centre, Novozymes, Beijing 100085, PR China 3CABI, Bakeham Lane, Egham, Surrey TW20 9TY, UK and Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK 4Cereal Disease Laboratory, U.S. Department of Agriculture, Agricultural Research Service, 1551 Lindig Street, St. Paul, MN 55108, USA 5CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands 6School of Environmental Sciences, University of Guelph, Guelph, Ontario, N1G 2W1, Canada 7International Fungal Research & Development Centre, The Research Institute of Resource Insects, Chinese Academy of Forestry, Bailongsi, Kunming 650224, PR China 8Landcare Research, Private Bag 92170, Auckland 1142, New Zealand 9BIOTEC Bioresources Technology Unit, National Center for Genetic Engineering and Biotechnology, NSTDA, 113 Thailand Science Park, Paholyothin Road, Khlong 1, Khlong Luang, Pathum Thani, 12120, Thailand 10Guizhou Academy of Agricultural Sciences, Guiyang, Guizhou 550006 PR China 11Hokuriku Research Center, National Agricultural Research Center, -

Clover Anthracnose Caused by Colletotrichum Trifolii
2 5 2 5 1.0 :; 1111128 1//// . :; 111112 8 11111 . Ww I~ 2.2 : I~ I 2.2 ~ W '"'w Ii£ w ~ ~ ~ ~ ~ 1.1 1.1 ...,a~ ... -- 111111.8 111111.8 '111111. 25 IIIII~ 111111.6 111111.25 111111.4 111111.6 MICROCOPY RESOLUTION TEST CH>XRT (MICROCOPY RESOLUTION TEST CHART NATIONAL BURlAU or SlANO;'RD~·196 H NATiONAL BUREAU OF SlA.NDARDS-1963·A ==========~=~:~========~TECHNICAL BULU'.TIN No. Z8~FEBRUARY. 19Z8 UNITlm:STATES DEPARTMENT OF AGRICULTURE WASHINGTON, D. C. CLOVER ANTHRACNOSE CAUSED BY COLLETOTRICHUM TRIFOLII By JOHN MONTEITH, Jr. ABsociate Pathologist, Office of Vegetable and Forage Diseases, Bureau of Plant Industry 1 CONTENTS Page Page rntroductlon _____________________ 1 The funguR in relntlon to anthrac ElIstol'Y and geogro9hlcal dlstrlbu- ., nos~('ontlnul'd. tlon___________________________ 3- Vlabillty Bnd longe't'lty ot DlY~ flost plnnts______________________ lIum and conidia ___________ 13 SYlI1ptom~ _______________________ 3 Dlsspmluution of conidla_______ 13 Inj lIty produced __________________ II Method of Infection and period The cau~,,1 ol'J~nnlsm--------------, 7 of Incubntlon_______________ 13 Tllxonomy __________.:.________ 7 Source of natural Infl'ctlon____ 14 l~olll t1ous____________________ 8 Environmental factors Influencing oc Spore germlnntlon ____________ 8 currence and progress of the dla- Cull ural characters ___________ 9 ease__________________________ 15 Helu tlon of tpllIpero ture to '.:'em peruture _________________ 15 growth 011 medIa ___________ \l hlolsture ____________________ 17 Errect of I\ght________________ -

Biological Activities of Trifolium Pratense: a Review
Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423) Volume 3 Issue 9 September 2019 Review Article Biological Activities of Trifolium Pratense: A Review Atiq-ur-Rehman1,2* 1University College of Pharmacy, University of the Punjab, Lahore, Pakistan 2Faculty of Pharmacy, The University of Lahore, Lahore, Pakistan *Corresponding Author: Atiq-ur-Rehman, Faculty of Pharmacy, The University of Lahore and University College of Pharmacy, University of the Punjab, Lahore Pakistan. Received: July 25, 2019; Published: August 16, 2019 Abstract Trifolium pratense is an important plant of the Legume family. It has drawn the attention of several researchers around the globe. This plant was traditionally used as forage or as soil improver is now seen as the plant containing vast therapeutic activities which include anti-oxidative, anti-cancer, neuroprotective, anti-hyperglycemic, anti-hyperlipidemic, osteoprotective and cardio protective properties. The therapeutic properties are shown in various in vivo, in vitro and ex vivo experiments. The review highlights the Tri- forium pratense basic knowledge its extraction, components and their actions, major activities possessed by plant along with their mechanisms. Trifolium plant is mainmajorly used in menopausal women to reduce the discomfort and menopausal effects such as moderate cancer causing cells. Various strategies were applied and the plant is still under study for further development in its effects. hot flushes and increase in breast density. The plant is also majorly responsible for preventing breast cancer and other apoptosis of Keywords: Trifolium Pratense; Cancer; Trifolium Introduction Family The genus Trifolium comprises of almost 240 species each re- It belongs to the family Fabeaceae leguminosae. markable for its agricultural and therapeutic effects. -

A Polyphasic Approach for Studying Colletotrichum
Online advance Fungal Diversity A polyphasic approach for studying Colletotrichum Cai, L.1*, Hyde, K.D.2,3, Taylor, P.W.J.4, Weir, B.S.5, Waller, J.M.6, Abang, M.M.7, Zhang, J.Z.8, Yang, Y.L.9, Phoulivong, S.3, Liu, Z.Y.9, Prihastuti, H.3, Shivas, R.G.10, McKenzie, E.H.C.5 and Johnston, P.R.5 1Novozymes China, No. 14, Xinxi Road, Shangdi, HaiDian, Beijing, 100085, PR China 2International Fungal Research & Development Centre, The Research Institute of Resource Insects, Chinese Academy of Forestry, Bailongsi, Kunming 650224, PR China 3School of Science, Mae Fah Luang University, Thasud, Chiang Rai 57100, Thailand 4BioMarka/Center for Plant Health, Melbourne School of Land and Environment, The University of Melbourne, Victoria 3010 Australia 5Landcare Research New Zealand Ltd, Private Bag 92170, Auckland Mail Centre, Auckland 1142, New Zealand 6CABI Europe UK, Bakeham Lane, Egham, Surrey, TW20 9TY. 7AVRDC-The World Vegetable Center, Regional Center for Africa, PO Box 10 Duluti, Arusha, Tanzania 8Institute of Biotechnology, College of Agriculture & biotechnology, Zhejiang University, Kaixuan Rd 258, Hangzhou 310029, PR China 9 Guizhou Academy of Agricultural Sciences, Guiyang, Guizhou 550006 P.R. China 10Plant Pathology Herbarium, Queensland Primary Industries and Fisheries, 80 Meiers Road, Indooroopilly 4068, Queensland, Australia Cai, L., Hyde, K.D., Taylor, P.W.J., Weir, B.S., Waller, J., Abang, M.M., Zhang, J.Z., Yang, Y.L., Phoulivong, S., Liu, Z.Y., Prihastuti, H., Shivas, R.G., McKenzie, E.H.C. and Johnston, P.R. (2009). A polyphasic approach for studying Colletotrichum. Fungal Diversity 39: 183-204. -

Notes on Currently Accepted Species of Colletotrichum
Mycosphere 7(8) 1192-1260(2016) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/si/2c/9 Copyright © Guizhou Academy of Agricultural Sciences Notes on currently accepted species of Colletotrichum Jayawardena RS1,2, Hyde KD2,3, Damm U4, Cai L5, Liu M1, Li XH1, Zhang W1, Zhao WS6 and Yan JY1,* 1 Institute of Plant and Environment Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, People’s Republic of China 2 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 3 Key Laboratory for Plant Biodiversity and Biogeography of East Asia (KLPB), Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, China 4 Senckenberg Museum of Natural History Görlitz, PF 300 154, 02806 Görlitz, Germany 5State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, 100101, China 6Department of Plant Pathology, College of Plant Protection, China Agricultural University, Beijing 100193, China. Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY 2016 – Notes on currently accepted species of Colletotrichum. Mycosphere 7(8) 1192–1260, Doi 10.5943/mycosphere/si/2c/9 Abstract Colletotrichum is an economically important plant pathogenic genus worldwide, but can also have endophytic or saprobic lifestyles. The genus has undergone numerous revisions in the past decades with the addition, typification and synonymy of many species. In this study, we provide an account of the 190 currently accepted species, one doubtful species and one excluded species that have molecular data. Species are listed alphabetically and annotated with their habit, host and geographic distribution, phylogenetic position, their sexual morphs and uses (if there are any known). -

The Colletotrichum Destructivum Species Complex – Hemibiotrophic Pathogens of Forage and field Crops
available online at www.studiesinmycology.org STUDIES IN MYCOLOGY 79: 49–84. The Colletotrichum destructivum species complex – hemibiotrophic pathogens of forage and field crops U. Damm1*, R.J. O'Connell2, J.Z. Groenewald1, and P.W. Crous1,3,4 1CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands; 2UMR1290 BIOGER-CPP, INRA-AgroParisTech, 78850 Thiverval-Grignon, France; 3Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa; 4Wageningen University and Research Centre (WUR), Laboratory of Phytopathology, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands *Correspondence: U. Damm, [email protected], Present address: Senckenberg Museum of Natural History Görlitz, PF 300 154, 02806 Görlitz, Germany. Abstract: Colletotrichum destructivum is an important plant pathogen, mainly of forage and grain legumes including clover, alfalfa, cowpea and lentil, but has also been reported as an anthracnose pathogen of many other plants worldwide. Several Colletotrichum isolates, previously reported as closely related to C. destructivum, are known to establish hemibiotrophic infections in different hosts. The inconsistent application of names to those isolates based on outdated species concepts has caused much taxonomic confusion, particularly in the plant pathology literature. A multilocus DNA sequence analysis (ITS, GAPDH, CHS-1, HIS3, ACT, TUB2) of 83 isolates of C. destructivum and related species revealed 16 clades that are recognised as separate species in the C. destructivum complex, which includes C. destructivum, C. fuscum, C. higginsianum, C. lini and C. tabacum. Each of these species is lecto-, epi- or neotypified in this study. Additionally, eight species, namely C. americae- borealis, C. antirrhinicola, C. bryoniicola, C. -

RED CLOVER This Plant May Become Weedy Or Invasive in Some Regions Or Habitats and May Displace Other Vegetation Trifolium Pratense L
Plant Guide Weediness RED CLOVER This plant may become weedy or invasive in some regions or habitats and may displace other vegetation Trifolium pratense L. if not properly managed. Consult your local NRCS Plant Symbol = TRPR2 Field Office, Cooperative Extension Service Office, or state natural resource or agriculture department Contributed by: Idaho Plant Materials regarding its status and use. Weed information is also available from the PLANTS web site at plants.usda.gov Description Trifolium pratense L., red clover, is an introduced biennial or short-lived perennial that grows as one of two types: medium (double-cut) or mammoth (single- cut). Red clover initiates growth from the plant crown. Plants have hollow, hairy stems and branches. Stem lengths of medium and mammoth types average 18 inches and 24-30 inches, respectively. Medium types have about 4 branches per stem; mammoth types have 6 branches per stem. USDA NRCS PLANTS Each leaf consists of a slender stalk bearing 3 leaflets. The taproot of red clover normally disintegrates in the second year and plants that Uses survive have developed secondary, extensively Red clover is the most widely grown of all the true branched roots. Flowers are borne in compact clovers and is the most important legume hay crop in clusters at the tips of the branches and are usually the northeastern United States. Red clover is rose-pink in color. The flower shape is similar to pea primarily used for hay, pasture, silage, and soil flowers except is more elongated and much smaller. improvement. It is a quick growing crop, easily Flower heads usually consist of up to 125 flowers. -

Conservation Plants Pocket ID Guide
About this guide.......... The purpose of this guide is to help you identify come commonly used conservation plants. Its color photos, line drawings and seed photos will help you make identifications. Also included are plant stand evaluation and recommended use charts. Keep this guide with you as long as you need it! Uses 1 2 Evaluating Stands Seeding success may not be obvious from visual observation. Use the chart below to determine whether your first-year stand is adequate. Lay a square-foot frame, or a circular frame with a 42.5-inch circumference on the ground. Count the number of seedlings within the frame, taking at least 10 counts for each 10 acres, in representative areas of the field. The table is based on pure stands; if a mixture of grass and legume is planted, reduce the numbers by the ratio of each species planted. Inadequate stands should be re-seeded. When a stand is judged to be between adequate and inadequate, it should be reevaluated after the second growing season. Warm-season grasses may need to be evaluated after the third growing season. 3 Seeds Early ID -- Seed is Key Grasses can be very difficult to identify in early growth stages. The seed may be the best identify aid. A seed retains its form and position in the ground through the seedling's early growth stages. To identify a seedling, carefully dig it up and compare it to photographs or actual seeds. 4 Plant Parts Conservation Plants The Elsberry Plant Materials Center The Elsberry Plant Materials Center is a 243-acre facility near Elsberry, Missouri.