Durian Floral Differentiation and Flowering Habit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calophyllum Inophyllum L
Calophyllum inophyllum L. Guttiferae poon, beach calophyllum LOCAL NAMES Bengali (sultanachampa,punnang,kathchampa); Burmese (ph’ông,ponnyet); English (oil nut tree,beauty leaf,Borneo mahogany,dilo oil tree,alexandrian laurel); Filipino (bitaog,palo maria); Hindi (surpunka,pinnai,undi,surpan,sultanachampa,polanga); Javanese (njamplung); Malay (bentagor bunga,penaga pudek,pegana laut); Sanskrit (punnaga,nagachampa); Sinhala (domba); Swahili (mtondoo,mtomondo); Tamil (punnai,punnagam,pinnay); Thai (saraphee neen,naowakan,krathing); Trade name (poon,beach calophyllum); Vietnamese (c[aa]y m[uf]u) Calophyllum inophyllum leaves and fruit (Zhou Guangyi) BOTANIC DESCRIPTION Calophyllum inophyllum is a medium-sized tree up to 25 m tall, sometimes as large as 35 m, with sticky latex either clear or opaque and white, cream or yellow; bole usually twisted or leaning, up to 150 cm in diameter, without buttresses. Outer bark often with characteristic diamond to boat- shaped fissures becoming confluent with age, smooth, often with a yellowish or ochre tint, inner bark usually thick, soft, firm, fibrous and laminated, pink to red, darkening to brownish on exposure. Crown evenly conical to narrowly hemispherical; twigs 4-angled and rounded, with plump terminal buds 4-9 mm long. Shade tree in park (Rafael T. Cadiz) Leaves elliptical, thick, smooth and polished, ovate, obovate or oblong (min. 5.5) 8-20 (max. 23) cm long, rounded to cuneate at base, rounded, retuse or subacute at apex with latex canals that are usually less prominent; stipules absent. Inflorescence axillary, racemose, usually unbranched but occasionally with 3-flowered branches, 5-15 (max. 30)-flowered. Flowers usually bisexual but sometimes functionally unisexual, sweetly scented, with perianth of 8 (max. -

The Miracle Resource Eco-Link
Since 1989 Eco-Link Linking Social, Economic, and Ecological Issues The Miracle Resource Volume 14, Number 1 In the children’s book “The Giving Tree” by Shel Silverstein the main character is shown to beneÞ t in several ways from the generosity of one tree. The tree is a source of recreation, commodities, and solace. In this parable of giving, one is impressed by the wealth that a simple tree has to offer people: shade, food, lumber, comfort. And if we look beyond the wealth of a single tree to the benefits that we derive from entire forests one cannot help but be impressed by the bounty unmatched by any other natural resource in the world. That’s why trees are called the miracle resource. The forest is a factory where trees manufacture wood using energy from the sun, water and nutrients from the soil, and carbon dioxide from the atmosphere. In healthy growing forests, trees produce pure oxygen for us to breathe. Forests also provide clean air and water, wildlife habitat, and recreation opportunities to renew our spirits. Forests, trees, and wood have always been essential to civilization. In ancient Mesopotamia (now Iraq), the value of wood was equal to that of precious gems, stones, and metals. In Mycenaean Greece, wood was used to feed the great bronze furnaces that forged Greek culture. Rome’s monetary system was based on silver which required huge quantities of wood to convert ore into metal. For thousands of years, wood has been used for weapons and ships of war. Nations rose and fell based on their use and misuse of the forest resource. -
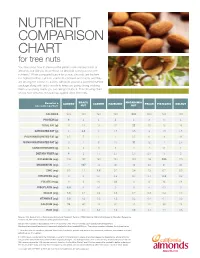
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Durio Zibethinus
1 The Draft Genome of Tropical Fruit Durian (Durio zibethinus) 2 1,2,3,4,5,6# 2,7 2,7 3 3 Bin Tean Teh , Kevin Lim *, Chern Han Yong *, Cedric Chuan Young Ng *, Sushma Ramesh 8,14,15,16 3 2,4, 7 9 10 4 Rao , Vikneswari Rajasegaran , Weng Khong Lim , Choon Kiat Ong , Ki Chan , Vincent Kin 11 12 8,14,15,16,17 2,4,7 13 5 Yuen Cheng , Poh Sheng Soh , Sanjay Swarup , Steven G Rozen , Niranjan Nagarajan , 1,2,4,5,13# 6 Patrick Tan 7 8 1 9 Thorn Biosystems Pte Ltd, Singapore 2 10 Program in Cancer and Stem Cell Biology, Duke-NUS Medical School, Singapore 3 11 Laboratory of Cancer Epigenome, Division of Medical Science, National Cancer Centre, Singapore 4 12 SingHealth/Duke-NUS Institute of Precision Medicine, National Heart Centre, Singapore 5 13 Cancer Science Institute of Singapore, National University of Singapore, Singapore 6 14 Institute of Molecular and Cellular Biology, Singapore 7 15 Centre for Computational Biology, Duke-NUS Medical School, Singapore 8 16 Department of Biological Sciences, National University of Singapore, Singapore 9 17 Lymphoma Genomic Translational Research Laboratory, National Cancer Centre, Singapore 10 18 Global Databank, Singapore 11 19 Verdant Foundation, Hong Kong 12 20 Samsoney Group, Malaysia 13 21 Genome Institute of Singapore, Singapore 14 22 Singapore Centre for Environmental Life Sciences Engineering, Nanyang Technological University, 23 Singapore 15 24 Metabolites Biology Lab, National University of Singapore, Singapore 16 25 NUS Synthetic Biology for Clinical and Technological Innovation, Life Sciences Institute, National 26 University of Singapore, Singapore 17 27 NUS Environmental Research Institute, National University of Singapore, Singapore 28 29 30 * Denotes equal contribution 31 32 # Address correspondence: [email protected] (B.T.T.) or [email protected] 33 (P.T.) 34 2 35 Abstract 36 Durian (Durio zibethinus) is a South East Asian tropical plant species, well-known for its hefty spine- 37 covered fruit and notorious sulfury and onion-like odor. -

UNECE Standard for Pine Nuts (DDP-12)
UNECE STANDARD DDP-12 concerning the marketing and commercial quality control of PINE NUT KERNELS 2013 EDITION UNITED NATIONS New York and Geneva, 2013 NOTE Working Party on Agricultural Quality Standards Working Party on Agricultural Quality Standards The commercial quality standards developed by the United Nations Economic Commission for Europe (UNECE) Working Party on Agricultural Quality Standards help facilitate international trade, encourage high-quality production, improve profitability and protect consumer interests. UNECE standards are used by Governments, producers, traders, importers and exporters, and other international organizations. They cover a wide range of agricultural products, including fresh fruit and vegetables, dry and dried produce, seed potatoes, meat, cut flowers, eggs and egg products. Any member of the United Nations can participate, on an equal footing, in the activities of the Working Party. For more information on agricultural standards, please visit our website http://www.unece.org/trade/agr/welcome.html. The new Standard for Pine Nut Kernels is based on document ECE/TRADE/C/WP.7/2013/31, reviewed and adopted by the Working Party at its sixty-ninth session. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the United Nations Secretariat concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of company -

Genetic Diversity of Hybrid Durian Resulted from Cross Breeding Between Durio Kutejensis and Durio Zibethinus Based on Random Amplified Polymorphic Dnas (Rapds)
American Journal of Molecular Biology, 2013, 3, 153-157 AJMB http://dx.doi.org/10.4236/ajmb.2013.33020 Published Online July 2013 (http://www.scirp.org/journal/ajmb/) Genetic diversity of hybrid durian resulted from cross breeding between Durio kutejensis and Durio zibethinus based on random amplified polymorphic DNAs (RAPDs) Tati Hariyati1, Joni Kusnadi1, Estri Laras Arumingtyas2 1Agroindustrial Biotechnology, Faculty of Agricultural Technology, University of Brawijaya, Malang, Indonesia 2Laboratory of Molecular Biology, Department of Biology, University of Brawijaya, Malang, Indonesia Email: [email protected], [email protected], [email protected] Received 17 April 2013; revised 17 May 2013; accepted 16 June 2013 Copyright © 2013 Tati Hariyati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT cal rain forests with the biggest biodiversity in the world, including genetic diversity of tropical fruits. Indonesia is One of the ways to improve the quality of Indonesian one of the eight centres of plant genetic diversity in the Durian is by utilizing germplasm diversity. Durio world, especially for tropical fruits like durian [1]. That zibethinus is the most cultivated durian in Indonesia, great amount of genetic diversity of Durio spp. in Indo- whereas Durio kutejensis is a unique durian cultivar nesia’s serves an essential base for plant breeding [2]. which has golden yellow fruit flesh without smell. Durian is one fruit commodities that has an important Crossbreeding of those two cultivars, in order to gen- economic value. -

Collection and Evaluation of Under-Utilized Tropical and Subtropical Fruit Tree Genetic Resources in Malaysia
J]RCAS International Symposium Series No. 3: 27-38 Session 1-3 27 Collection and Evaluation of Under-Utilized Tropical and Subtropical Fruit Tree Genetic Resources in Malaysia WONG, Kai Choo' Abstract Fruit tree genetic resources in Malaysia consist of cultivated and wild species. The cul tivated fruit trees number more than 100 species of both indigenous and introduced species. Among these fruits, some are popular and are widely cultivated throughout the country while others are less known and grown in small localized areas. The latter are the under-utilized fruit species. Apart from these cultivated fruits, there is also in the Malaysian natural forest a diversity of wild fruit tree species which produce edible fruits but are relatively unknown and unutilized. Many of the under-utilized and unutilized fruit species are known to show economic potential. Collection and evaluation of some of these fruit tree genetic resources have been carried out. These materials are assessed for their potential as new fruit trees, as sources of rootstocks for grafting and also as sources of germplasm for breeding to improve the present cultivated fruit species. Some of these potential fruit tree species within the gen era Artocarpus, Baccaurea, Canarium, Dimocarpus, Dialium, Durio, Garcinia, Litsea, Mangif era, Nephelium, Sa/acca, and Syzygium are highlighted. Introduction Malaysian fruit tree genetic resources comprise both cultivated and wild species. There are more than 100 cultivated fruit species of both major and minor fruit crops. Each category includes indigenous as well as introduced species. The major cultivated fruit crops are well known and are commonly grown throughout the country. -

Morphological Description of Jogorogo Mangosteen (Garcinia Mangostana L.)
Journal of Biotechnology and Biodiversity, April 2010; 1(1): 20-25 ISSN: 2087-0183 RESEARCH Morphological description of Jogorogo Mangosteen (Garcinia mangostana L.) Endang Yuniastutia* aDepartment of Agrotechnology, Faculty of Agriculture, Sebelas Maret University, Jl. Ir. Sutami no 36A, Surakarta 57126, Indonesia Received : 14 December 2009 Accepted: 2 February 2010 Abstract This research aimed to obtain phenotypic information based on morphological character of Jogorogo Mangosteen (Garcinia mangostana L.). This research was conducted with direct observation through primary and secondary data recording, and documenting parts of Jogorogo Mangosteen plant specifically, that was, in vegetative part: stalk and leave, as well as generative part: flower, fruit and seed. Jogorogo Mangosteen may reach hundreds years of life span, it had an average height of 9 meters, stalk diameter of 1 meter and crown diameter of 6 meter. The tree crown of Jogorogo Mangosteen plant was triangular in shape, with horizontal and irregular branching pattern and various densities. The leaves of Jogorogo Mangosteen were elliptic. The tip of the leaf was pointed, the base of the leaf was blunt, and the leaf edge was flat with the smooth and shining surface. The flower of Jogorogo Mangosteen was a hermaphrodit and a perfect flower. The fruit was small with 59 grams weight/flower with 4.5 cm long and 4.45 cm wide. The fruit was purple- blackish with the continuous fruit ripening with high fruit bearing level. The Jogorogo Mangosteen fruit was sweet with a little yellow sap. 1-2 seeds were formed in every Jogorogo Mangosteen fruit with 1.6 cm long, 0.8 cm wide and 2.75 thick. -

Various Terminologies Associated with Areca Nut and Tobacco Chewing: a Review
Journal of Oral and Maxillofacial Pathology Vol. 19 Issue 1 Jan ‑ Apr 2015 69 REVIEW ARTICLE Various terminologies associated with areca nut and tobacco chewing: A review Kalpana A Patidar, Rajkumar Parwani, Sangeeta P Wanjari, Atul P Patidar Department of Oral and Maxillofacial Pathology, Modern Dental College and Research Center, Indore, Madhya Pradesh, India Address for correspondence: ABSTRACT Dr. Kalpana A Patidar, Globally, arecanut and tobacco are among the most common addictions. Department of Oral and Maxillofacial Pathology, Tobacco and arecanut alone or in combination are practiced in different regions Modern Dental College and Research Centre, in various forms. Subsequently, oral mucosal lesions also show marked Airport Road, Gandhi Nagar, Indore ‑ 452 001, Madhya Pradesh, India. variations in their clinical as well as histopathological appearance. However, it E‑mail: [email protected] has been found that there is no uniformity and awareness while reporting these habits. Various terminologies used by investigators like ‘betel chewing’,‘betel Received: 26‑02‑2014 quid chewing’,‘betel nut chewing’,‘betel nut habit’,‘tobacco chewing’and ‘paan Accepted: 28‑03‑2015 chewing’ clearly indicate that there is lack of knowledge and lots of confusion about the exact terminology and content of the habit. If the health promotion initiatives are to be considered, a thorough knowledge of composition and way of practicing the habit is essential. In this article we reviewed composition and various terminologies associated with areca nut and tobacco habits in an effort to clearly delineate various habits. Key words: Areca nut, habit, paan, quid, tobacco INTRODUCTION Tobacco plant, probably cultivated by man about 1,000 years back have now crept into each and every part of world. -

Allowable Vs. Unallowable Items
Allowable vs. Unallowable Items ALLOWABLE UNALLOWABLE ITEMS ITEMS Processed or preserved fruits and vegetables Whole or sliced fresh fruits and vegetables (i.e., canned, frozen, vacuum-packed or Pre-sliced, pre-cut, FRESH produce dried) Condiments—lemons, limes, and/or chili powder Fruit leather or jellied fruit can be used as a condiment to be served with Dips for fruit or cottage cheese vegetables Fruit or vegetable juice Vegetable dips—are allowed if they are low-fat yogurt-based, or other low-fat or non-fat dips Smoothies All dip serving sizes cannot exceed 1–2 Grapples tablespoons Trail mixes or nuts Salsas are allowable, as a prepared item; Fruit/vegetable pizza however, they must be accompanied with Fruit that has been injected with flavorings nutrition education Carbonated fruit Fresh coconut Most non-food items, except those allowed Fresh vegetables that are cooked, must be limited under supplies and administrative/operational to service once-a-week and always as part of a costs nutrition education lesson Sending fruits and vegetables home Examples: Apples, Bananas, Apricots, Pineapple, Serving fruits and vegetables outside the Mango, Broccoli, Berries, Melons, Carrots, Grapes, normal school hours Cucumbers, Kiwi, Kumquats, Mushrooms, Onions, Popcorn Oranges, Peaches, Pears, Plums, Pomegranates, Candies or marshmallows Radishes, Beets, Leafy greens, Kale, Spinach, Sweet Potato, Jicama, Ackee, Rambutan, Durian, Mangosteen, Cherimoya, Pepino, Papaya, Artichoke, Asparagus, Green Beans, Peppers, Okra, Squash This institution is an equal opportunity provider. . -

COMPARATIVE LIFE HISTORY of COCONUT SCALE INSECT, Aspidiotus Rigidus Reyne (HEMIPTERA: DIASPIDIDAE), on COCONUT and MANGOSTEEN
J. ISSAAS Vol. 25, No. 1: 123-134 (2019) COMPARATIVE LIFE HISTORY OF COCONUT SCALE INSECT, Aspidiotus rigidus Reyne (HEMIPTERA: DIASPIDIDAE), ON COCONUT AND MANGOSTEEN Cris Q. Cortaga, Maria Luz J. Sison, Joseph P. Lagman, Edward Cedrick J. Fernandez and Hayde F. Galvez Institute of Plant Breeding, College of Agriculture and Food Science, University of the Philippines Los Baños, College, Laguna, Philippines 4031 Corresponding author: [email protected] (Received: October 3, 2018; Accepted: May 19, 2019) ABSTRACT The devastation of millions of coconut palms caused by outbreak infestation of the invasive Coconut Scale Insect (CSI) Aspidiotus rigidus Reyne, has posed a serious threat to the industry in the Philippines. The life history of A. rigidus on coconut and mangosteen was comparatively studied to understand the effects of host-plant species on its development, to investigate potential host-suitability factors that contributed to its outbreak infestation, and to gather baseline information on the development and characteristics of this pest. The study was conducted at the Institute of Plant Breeding, College of Agriculture and Food Science, University of the Philippines Los Baños. Insect size (body and scale) was not significantly different on both hosts during egg, crawler, white cap, pre-second and second instar stages, as well as during male pre-pupal, pupal and adult stages. The female third instars and adults, however, were bigger on mangosteen than on coconut. At the end of second instar, sexual differentiation was very visible wherein parthenogenic females further undergone two developmental stages: third instar and adults that feed permanently on the leaves. Males undergone three stages: pre- pupa, pupa and winged adults. -

Virtually Bringing the Nut & Dried Fruit Sector Together
Edition 81. Nº 3 November 2020 INC ONLINE CONFERENCE Virtually Bringing the Nut & Dried Fruit Sector Together p. 57 www.nutfruit.org November 2020 | NUTFRUIT November 2020 | NUTFRUIT Edition 81. Nº 3 November 2020 The INC is the international umbrella organization for the nut and dried fruit industry and the source for information on health, nutrition, statistics, food safety, and international standards and regulations regarding nuts and dried fruits. BOARD OF TRUSTEES Michael Waring - Chairman Business News 9 INC Congress 54 MWT Foods, Australia Ashok Krishen - 1st Vice Chairman 9 Partnership Besana-Importaco 54 Dubai, INC XXXIX World Nut and Dried Olam International Limited, Singapore Fruit Congress Pino Calcagni - 2nd Vice Chairman 10 PepsiCo Targets 100% Renewable Besana Group, Italy Electricity Globally Riccardo Calcagni Besana Group, Italy 11 Danone’s Alpro Celebrates 40 Years INC News 57 Bill Carriere Carriere Family Farms, USA 12 Creamy, Crunchy, Chewy: Introducing 57 INC Online Conference Karsten Dankert Nature Valley Packed, a New Sustained Max Kiene GmbH, Germany Energy Bar 60 INC Academia: The Best Training Program in Roby Danon the Nut and Dried Fruit Industry Voicevale Ltd, UK Cao Derong 62 INC Webinars China Chamber of Commerce, China Gourmet 14 Joan Fortuny Borges Agricultural & Industrial Nuts (BAIN), Spain 63 Trend Research: International Market Giles Hacking 14 Carme Ruscalleda, Barcelona, Spain Opportunities CG Hacking & Sons Limited, UK Mike Hohmann 64 Real Power for Real People: Boost your The Wonderful Company,