Multiple Myeloma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Borough Profile 2020 Warrington
Borough profile 2020 Warrington 6 4 3 117 122 118 115 9 5 19 120 7 Warrington Wards 2 13 1 1. Appleton 12. Latchford West 110 11 12 2. Bewsey & Whitecross 13. Lymm North & Thelwall 1 14 3. Birchwood 14. Lymm South 4. Burtonwood & Winwick 15. Orford 116 21 5. Chapelford & Old Hall 16. Penketh & Cuerdley 8 6. Culcheth, Glazebury & Croft 17. Poplars & Hulme 7. Fairfield & Howley 18. Poulton North 8. Grappenhall 19. Poulton South 1 9. Great Sankey North & Whittle Hall 20. Rixton & Woolston 10. Great Sankey South 21. Stockton Heath 11. Latchford East 22. Westbrook Produced by Business Intelligence Service Back to top Contents 1. Population of Warrington 2. Deprivation 3. Education - Free School Meals (FSM) 4. Education - Special Educational Needs (SEN) 5. Education - Black Minority Ethnic (BME) 6. Education - English as an Additional Language (EAL) 7. Education - (Early Years aged 4/5) - Early Years Foundation Stage: Good Level of Development (GLD) 8. Education - (End of primary school aged 10/11) – Key Stage 2: Reading, Writing and Maths 9. Education (end of secondary school aged 15/16) – Key Stage 4: Progress 8 10. Education (end of secondary school aged 15/16) – Key Stage 4: Attainment 8 11. Health - Life expectancy 12. Health - Low Birthweight 13. Health - Smoking at time of delivery 14. Health - Overweight and obese reception children 15. Health - Overweight and obese Year 6 children 16. Children’s Social Care – Children in Need 17. Adult Social Care – Request for Support from new clients 18. Adult Social Care – Sequel to the Requests for Support 19. Adult Social Care – Number of clients accessing Long Term Support 20. -

87 York Road Brigg North Lincolnshire DN20 8DX Asking Price
The Largest Independent Auction, Estate & Letting Agency in the Region 87 York Road Brigg North Lincolnshire DN20 8DX . Good sized family home in popular location . Lounge with sun room off . Breakfast kitchen & utility . Four bedrooms & bathroom . Off road parking & garage . EPC RATING : D Asking Price: £159,000 Further information and viewings: DDM Residential - Brigg Office - 01652 653666 DESCRIPTION A four bedroom detached family home situated in a popular residential area of Brigg with easy access to the town centre and local schools. The property is decorated to a high standard throughout and briefly comprises entrance hall, lounge with sun room off, breakfast kitchen, utility and cloakroom. To the first floor there are four bedrooms and a family bathroom. Having off road parking, garage and gardens to the front and rear. A good sized family home in excellent location. ACCOMMODATION ENTRANCE HALL uPVC double glazed entrance door, cornice to ceiling, uPVC double glazed window to the front aspect, radiator, stairs to first floor. SITTING ROOM 15' 1'' x 11' 5'' (4.59m x 3.49m) Cornice to ceiling, uPVC double glazed bay window to the front aspect, traditional style painted fire surround with tiled inset and hearth to flame effect electric fire, radiator. SUN ROOM/DINING AREA 14' 0'' x 8' 3'' (4.26m x 2.51m) Cornice to ceiling, uPVC double glazed windows and roof, combination heater/air conditioning unit, tiled floor and uPVC double glazed French doors to the rear garden. BREAKFAST KITCHEN 15' 0'' x 11' 5'' (4.58m x 3.48m) Inset ceiling spot lights, uPVC double glazed windows to the front and rear aspects, range of base and wall mounted units with contrasting beech effect work surfaces, inset one and a half bowl composite sink and drainer with mixer tap, integrated dishwasher and fridge freezer, tiled splashback, black ash effect flooring, archway to: REAR LOBBY Understairs storage cupboard, radiator, black ash effect flooring. -

Changes in the Ethnic Diversity of the Christian Population in England
National Census 2001 and 2011 Changes in the Ethnic Diversity of the Christian Population in England between 2001 and 2011 East Midlands Region Council for Christian Unity 2014 CONTENTS Foreword from the Chair of the Council for Christian Unity Page 1 Summary and Headlines Page 2 Introduction Page 2 Christian Ethnicity - Comparison of 2001 and 2011 Census Data Page 5 In England Page 5 By region Page 8 Overall trends Page 24 Analysis of Regional data by local authority Page 27 Introduction Page 27 Tables and Figures Page 28 Annex 2 Muslim Ethnicity in England Page 52 Census 2001/2011 East Midlands CCU(14)C3 Changes in the Ethnic Diversity of the Christian Population in England between 2001 and 2011 Foreword from the Chair of the Council for Christian Unity There are great ecumenical, evangelistic, pastoral and missional challenges presented to all the Churches by the increasing diversity of Christianity in England. The comparison of Census data from 2001 and 2011about the ethnic diversity of the Christian population, which is set out in this report, is one element of the work the Council for Christian Unity is doing with a variety of partners in this area. We are very pleased to be working with the Research and Statistics Department and the Committee for Minority Ethnic Anglican Affairs at Church House, and with Churches Together in England on a number of fronts. We hope that the set of eight reports, for each of the eight regions of England, will be a helpful resource for Church Leaders, Dioceses, Districts and Synods, Intermediate Ecumenical Bodies and local churches. -

NHS North Lincolnshire Clinical Commissioning Group 2014-15
NHS North Lincolnshire Clinical Commissioning Group 2014-15 Annual Report Contents 1. Introduction……………………………………………………………………………………………………………….…… 1 2. Welcome from the CCG Chair and Chief Officer ………………………………………………………….….. 3 3. Member Practices Introduction …………………………………………………………………………………….. 5 4. Strategic Report 4.1 About the CCG and its community ……………………………………………………………………….….. 9 4.2 The CCG business model ………………………………………………………………………………………….. 11 4.3 The CCG strategy ……………………………………………………………………………………………………… 13 4.4 Achievements this year ……………………………………………………………………………………………. 15 4.5 Risks facing the CCG …………………………………………………………………………………………………. 18 4.6 CCG performance …………………………………………………………………………………….…………..….. 19 4.7 The wider context in which the CCG operates …………………………………………………….……. 20 4.8 The CCG’s year-end financial position ………………………………………………………………………. 21 4.9 Going concern ………………………………………………………………………………………………….…….... 22 4.10 Assurance Framework ……………………………………………………………………………………………. 22 4.11 Sustainability Report ………………………………………………………………………………………………. 27 4.12 Equality and Diversity Report …………………………………………………………………………………. 32 4.13 Key Performance indicators ……………………………………………………………………………………. 36 4.14 Accountable Officer Declaration……………………………………………………………………………… 36 5. The Members’ Report 5.1 The Governing Body ………………………………………………………………………………………………… 37 5.2 Member practices of the CCG ……………………………………………………………………………..…. 38 5.3 Audit Group …………………………………………………………………………………………………………….. 41 5.4 Committee and Sub-committee membership and Declarations of interest -

STP: Latest Position Developing and Delivering the Humber, Coast and Vale Sustainability and Transformation Plan July 2016 Who’S Involved?
STP: Latest position Developing and delivering the Humber, Coast and Vale Sustainability and Transformation Plan July 2016 Who’s involved? NHS Commissioners Providers Local Authorities East Riding of Yorkshire CCG Humber NHS Foundation Trust City of York Council Hull CCG North Lincolnshire and Goole East Riding of Yorkshire Council NHS Foundation Trust Hull City Council North Lincolnshire CCG Tees, Esk and Wear Valleys North East Lincolnshire CCG NHS Foundation Trust North Lincolnshire Council City Health Care Partnerships North East Lincolnshire Council Scarborough and Ryedale CCG CIC Vale of York CCG Hull and East Yorkshire North Yorkshire County Council Hospitals NHS Trust Navigo Rotherham, Doncaster and South Humber NHS Foundation Trust York Teaching Hospital NHS Foundation Trust Our footprint Population characteristics 1.4 million population 23% live in the most deprived areas of England Diverse rural, urban and coastal communities Huge variation in health outcomes Three main acute providers Hull and East Yorkshire Hospitals NHS Trust North Lincolnshire and Goole NHS Foundation Trust York Teaching Hospital NHS Foundation Trust Drivers for change • Financial pressures across the system – significant and growing financial deficit across the system, doing nothing is not an option • Average life expectancies across the footprint range widely • Smoking, child and adult obesity rates and excessive alcohol consumption are above (some significantly) national averages • Stroke – our premature mortality rates from stroke are higher than average -

02/07/2018 – 30/06/2022 Document 1 of 2 Version 4 – May 2019
02/07/2018 – 30/06/2022 Document 1 of 2 Version 4 – May 2019 1 Provider Page ABC Counselling, Play Therapy & Family Solutions C.I.C. 3 ‐ 6 A Time 4 You Child and Family Psychological Services Ltd 7 – 10 Alliance Psychological Services Limited 11 – 14 Barnardo’s 15 – 17 Brook 18 – 21 Children North East 22 – 24 Doorways 25 – 27 MATRIX Neurological 28 – 31 Rainbows End Coaching 32 – 34 Redcar and Cleveland Mind 35 – 37 Tees Valley Therapies 38 – 39 The Junction 40 – 42 The Link CIC 43 – 46 The Wellbeing Centre (Saltburn) CIC 47 – 48 Volunteering Matters 49 – 52 Intervention costs are outlined in a separate document, Schools and RCBC staff can obtain a copy of this by emailing: becky.dale@redcar‐cleveland.gov.uk 2 Name of organisation: ABC Counselling, Play Therapy & Family Solutions C.I.C. Contact name for Peter Lowe MBE or Karen Lowe referrals/enquiries: Email [email protected] ‐ [email protected] Contact Details: Tel 01642 913060 mobile : 07971072789 ‐ 07939922194 Website: www.abccounsellingservices.com Overview of Partnership working services/interventions: ABC Counselling, Play Therapy & Family Solutions and A Time 4 You Psychological Service partnered together to expand reach and share professional expertise and have provided professional counselling and play therapy across over 60 schools in the Tees valley area. We have built up excellent working relationships with key staff, children and importantly Parents to support children and young people to build resilience and use coping strategies through a range of therapeutic interventions, both short and long term, including group work. What we provide Support can be offered to any child or young person with emotional, behavioural or mild to moderate mental health concerns. -
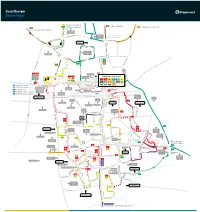
Scunthorpe Route Map
Scunthorpe Route Map 7 Continues as service 8 60 to Burton, Whitton 8 Continues as service 7 350 to Winterton, Barton, Hull 60 to Flixborough, Burton, Whitton Skippingdale Retail Park Ferry Road West Foxhills Outbound morning journeys Phoenix Parkway Industrial Estate Inbound evening journeys L Orbital Rd u rg n Mannabe Way e 8 b 7 u Crosby r g 7 8 W res N C a eedw l Portman Rd o p el y r a F S m er 8 ry CrosbyAv R n oa b d y W e Outwood R s d t Academy The Poplars Foxhills Foxhills Rd Warren Rd 8 Ferry Rd Rd Ferry 4 D 60 e w 8 350 Frodingham Rd s Winterton Rd 60 b u r y A A1077 Orbital Rd v UTC Brigg Rd Scotter Rd Lidl 1 1a Vivian Avenue Marsden Dv Sainsburys Gallagher Stanley Rd 1 1a Scunthorpe Town Centre Retail Park 7 Burn Rd St Lawrences Doncaster Rd 7 35 Academy Bus Station Tesco 1 1a 3 4 x4 7 7 90 60 HiltonAve 8 9 12 35 60 90 Doncaster Rd 360 361 399 350 35 to Amcotts, Crowle Doncaster Rd Cli Gardens d Moors Rd Mary St R d R 90 to Amcotts, Crowle North Lincolnshire r on e Kingsway ati Shopping Park v St to Crowle, Goole o Gardens 360 s Glanford Park 9 Hospital Scunthorpe Scunthorpe ol 361 to Westwoodside, Doncaster B United FC Minster Rd Church Lane Rowland Rd Kingsway 9 399 to Westwoodside, Doncaster Golf Course Midland Brumby Wood Lane Industrial Lodge Moor Brumby Wood Lane eck Rd Estate Steel Scotter Rd B Cottage Works A18 Kingsway The Brumby Pods 1 Wood Rd Ashby Cemetery Rd Quibell Park Brumby Frodingham Central Park UCNL Lilac A Crematorium ve Warwick Rd S a North n The Common Outward d Lindsey h College Academy o P 1a u lymouth -

Planning for Renewable Energy Development | Supplementary Planning Document | November 2011 Page | 1 Policy 7 - Community Impact
PlanningPlanning forfor RenewableRenewable EnergyEnergy DevelopmentDevelopment Spatial Planning Regeneration and Planning North Lincolnshire Council Supplementary Pittwood House Planning Document Ashby Road Scunthorpe November 2011 DN16 1AB Contents 1 Introduction ...........................................................................................................................................................................3 2 What is Renewable Energy? ...................................................................................................................................................5 3 North Lincolnshire & Renewable Energy ...............................................................................................................................7 On-Shore Wind ...................................................................................................................................................................7 Offshore Wind .....................................................................................................................................................................8 Carbon Capture and Storage ..................................................................................................................................................9 Biofuels ...............................................................................................................................................................................9 Biomass ............................................................................................................................................................................10 -

Progress in the Hull and Humber Ports City Region Statistical Update – March 2010
Progress in the Hull and Humber Ports City Region Statistical Update – March 2010 Introduction Welcome to Progress in the Hull and Humber Ports City Region 2010, produced by Humber Economic Partnership (HEP) on behalf of and with support by Yorkshire Futures. This document provides a statistical update on the performance of the city region and is a light touch review to support work on the Functional Sub-Regional and statutory Local Economic Assessments. It updates the Headline and High Level indicators outlined in the Integrated Regional Framework. There are three Headline Indicators and 35 high-level indicators, against which progress is measured. Data for some of these are unavailable at below regional geographies, so to add value, proxy indicators have been included where possible or appropriate. The cut off point for data collection was January 2010. The IRF indicators were first reported by HEP in the three-year major review Progress in the Hull and Humber Ports City Region 2009, which can be accessed via this link: http://www.humberep.co.uk/pageDocuments.php?section=64&search=2009&searchon=year In keeping with former reports, the chapters are ordered as follows 1. Population and Settlements 2. Economy 3. Quality of Life 4. High Quality Environments 5. Infrastructure 6. Education and Skills 7. Governance and Civic Participation Building on the work undertaken in this, and to complement the forthcoming Functional Sub-Regional and Local Economic Assessments, two new geographies have been reported where possible: the South Bank, covering the areas of North East Lincolnshire and North Lincolnshire, and the North Bank, covering the East Riding of Yorkshire and Kingston upon Hull. -

Duke House, Queensway Court, Arkwright Way, Scunthorpe, North Lincolnshire, DN16 3RT Offices for Sale/To Let 213.6 Sq M (2,300 Sq Ft) to 427.2 Sq M
Duke House, Queensway Court, Arkwright Way, Scunthorpe, North Lincolnshire, DN16 3RT Offices For Sale/To Let 213.6 sq m (2,300 sq ft) to 427.2 sq m (4,600 sq ft) approx. (3,218 sq ft) Approx. Chartered Surveyors & Commercial Property Consultants 01724 282278 pph-commercial.co.uk Summary The property is of traditional cavity brick/block Rent • Modern two storey office premises with designated car construction beneath a pitched concrete interlocking From £14,000 per annum, exclusive of rates, VAT (if applicable) parking from 213.6 sq m (2,300 sq ft) to 427.28 sq m (4,600 tiled roof with powder coated aluminium double glazed and all other outgoings. The rent is to be paid quarterly in sq ft) approx. windows, certain of which benefit from internal security advance. shutters. • Popular location close to A18 and M180. Lease Term • Available as a whole or as two separate suites. Internally, the accommodation provides two ground floor The premises are available by way of a new lease(s). Terms by entrances/receptions with a range of private/general negotiation. Location offices over ground and first floor together with kitchens Scunthorpe is the principle town within North Lincolnshire and WC facilities. Repairs and Insurance which has a population in excess of 152,000 with a much The Tenant(s) will be responsible for all repairs and decorations larger catchment area within a 30 minute drive. Externally, there are 12 designated car parking spaces with to the demised premises together with the reimbursement of the building. There are also a number of undesignated car Queensway Court is located approximately 3 miles South the insurance premium. -

North Lincolnshire Council
North Lincolnshire Council Adult Social Care Provider Public Report Bristol Road & Gervase Street Home Leader: Janice Price Creative Support c/o Haldenby House Unit 2-3 Berkley Business Centre, Doncaster Road, Scunthorpe. DN15 7DQ Registered Manager: Diane Ewen Introduction: Creative Support provides staff to deliver care and support to people who live in supported living projects and houses. The aim of the service is to provide people with the support they need to live as independently as possible. Type of provider: Care in your home and supported living Specialisms: .Learning disability Number of flats/rooms: 2 houses supporting 5 people Menu of services available: supported living Pricing structure/Fees of services available: individually assessed Star rating by residents: 5 stars What people told us: As part of our annual checks residents are asked how they feel about their lifestyles and what they think about the care they receive. At the time of the survey, a discussion was held with the 5 residents. This is what people told us: Everyone agreed that their health was good and they can all access their GP and hospital easily with staff assistance Everyone felt safe and secure in their home They were involved with decisions affecting their lives They agreed that they all felt valued by the people around them and could talk confidentially with staff They agreed that they enjoyed living at Bristol Road and liked the freedom they had We also received comments such as: “nothing at all” could improve the service Connect to Support -

Total Transport: Feasibility Report & Pilot Review
Total Transport: feasibility report & pilot review Moving Britain Ahead December 2017 The Department for Transport has actively considered the needs of blind and partially sighted people in accessing this document. The text will be made available in full on the Department’s website. The text may be freely downloaded and translated by individuals or organisations for conversion into other accessible formats. If you have other needs in this regard please contact the Department. Department for Transport Great Minster House 33 Horseferry Road London SW1P 4DR Telephone 0300 330 3000 Website www.gov.uk/dft General enquiries: https://forms.dft.gov.uk © Crown copyright 2017 Copyright in the typographical arrangement rests with the Crown. You may re-use this information (not including logos or third-party material) free of charge in any format or medium, under the terms of the Open Government Licence. To view this licence, visit http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or e-mail: [email protected] Where we have identified any third-party copyright information you will need to obtain permission from the copyright holders concerned. Contents Introduction 4 Executive summary 5 1. What is Total Transport? 6 2. The pilots 8 The bidding process 8 Participation 8 Benefits and outcomes 10 Barriers 15 3. Lessons learned 17 Annex A: List and description of pilot schemes 20 Annex B: Continuation of pilot schemes 23 Annex C: Case studies 25 Devon County Council 25 Lincolnshire County Council 27 3 Introduction 1 In spring 2015, the Department for Transport (DfT) held a competition to allocate funds for pilot schemes based on the principles of Total Transport.