Organic Chemistry Laboratory Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -

Site-Directed Spin Labeling of Large Riboswitches Using Click Chemistry
Site-Directed Spin Labeling of Large Riboswitches Using Click Chemistry Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Mark Kerzhner aus Winnyzja, Ukraine Bonn 2018 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Gutachter: Prof. Dr. Michael Famulok 2. Gutachter: Prof. Dr. Olav Schiemann Tag der Promotion: 25.10.2018 Erscheinungsjahr: 2019 Parts of this thesis have been published in: Kerzhner, M.; Abdullin, D.; Więcek, J.; Matsuoka, H.; Hagelueken, G.; Famulok, M.; Schiemann, O., Post-synthetic Spin-Labeling of RNA through Click Chemistry for PELDOR Measurments, Chem. Eur.J. 2016, 22, 12113 –12121. Kerzhner, M.; Matsuoka, H.; Wuebben, C.; Famulok, M.; Schiemann, O., High- Yield Spin Labeling of Long RNAs for Electron Paramagnetic Resonance Spectroscopy, Biochemistry, 2018, 57, 2923–2931. Danksagung An erster Stelle möchte ich Herrn Prof. Dr. Michael Famulok danken, dass er mir die Chance ermöglicht hat, an diesem anspruchsvollen aber hochinteressanten Projekt zu arbeiten. Außerdem bin ich ihm sehr dankbar, dass er mir über die Jahre sein Vertrauen geschenkt hat. Großer Dank gilt außerdem dem Kooperationspartner Herrn Prof. Dr. Olav Schiemann, ohne dessen Unterstützung die Umsetzung dieses Projektes nicht möglich gewesen wäre. Er und seine Arbeitsgruppe haben entscheidende Beiträge zu den erzielten Ergebnissen dieser Arbeit geleistet. Allen Mitgliedern meiner Prüfungskommission danke ich für die Begutachtung dieser Dissertation. Ich danke meinen Kooperationspartnern aus der Arbeitsgruppe von Herrn Prof. Dr. Olav Schiemann. Bei Dr. Dinar Abdullin, Dr. Hideto Matsuoka und Dr. Andreas Meyer bedanke ich mich herzlich für die zahlreichen PELDOR-Messungen sowie Hilfe bei verschiedenen Fragestellungen. -

Viruses at Glycocalyx Barriers SI Bioarxiv
Glycocalyx crowding with synthetic mucin mimetics strengthens interactions between soluble and virus-associated lectins and cell surface glycan receptors Authors: aDaniel J. Honigfort, b, † Meghan O. Altman, bPascal Gagneux, and Kamil Godula*,a Author Affiliation: aDepartment of Chemistry and Biochemistry, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0358, USA bDepartment of Pathology, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0687, USA Supporting Information 1 Table of contents: Instrumentation and reagents. ............................................................................................................ 4 Synthesis of propargyl glycosides. ........................................................... Error! Bookmark not defined. Scheme S1. Preparation of β-propargyl glycosides via the Schmidt glycosylation.Error! Bookmark not defined. Synthesis of glycopolymers GP-S/M/L ............................................................................................... 5 Scheme S2. Synthesis of Glycopolymers through iterative CuAAc click strategy. ................................ 5 General Procedure for the preparation of poly(epichlorohydrin) (P1). ............................................ 5 General Procedure for the end-functionalization of poly(epichlorohydrin) (P2). ............................ 6 General Procedure for the preparation of poly(Glycidyl Azide) (P3). .............................................. 6 General procedure for the preparation of Glycopolymers -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -

Chem 1140; Techniques for Handling Air-Sensitive Compounds
P. Wipf 1 Chem 1140 Chem 1140; Techniques for Handling Air-Sensitive Compounds • Introduction • The Glove Box • Schlenk Techniques • Drying and Degassing Solvents What are Air-Sensitive Compounds? Materials which oxidize, decompose or even explode under the influence of oxygen or moisture. • Pyrophoric Compounds Metal alkyls and aryls e.g. RMgX, RLi, RNa,R3Al, R2Zn Metal carbonyls e.g. Ni(CO)4, Fe(CO)5, Co2(CO)8 Alkali metals e.g. Na, K, Cs Metal powders e.g. Al, Co, Fe, Mg, Pd, Pt, Zn Metal hydrides e.g. NaH, KH, LiAlH4 Hydrides e.g. B2H6, PH3, AsH3 Boranes, phosphines, arsenes, etc. e.g. Et3B, R3P, R3As • Chemicals which react violently with water Metal hydrides, metal amides (NaNH2), metal alkyls and aryls, metals, metal powders, hydrides, many main group halides (BCl3, BF3, AlCl3, PCl3, SiCl4), inorganic acid halides (POCl3, SOCl2), low molecular weight organic acid halides and anhydrides. Glove boxes and Schlenk techniques do NOT protect from explosive or shock sensitive materials or mixtures!!! Also, they only provide limited protection from toxic compounds. P. Wipf 2 Chem 1140 The Glove Box • The best way to keep things away from atmospheric oxygen and water is to work in a fully enclosed “bench top,” containing an “inert atmosphere,” which one could reach into with gloves. Such a device is called a “glove box” or a “dry box”. There are also cheap “glove bags”, bags you can fill with inert gas and reach into with attached gloves. The Glove Box A glove box has four important components: 1. The actual “box” is a large aluminum chamber with a plastic front window and two impressive looking gloves. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -
Minimum Reflux Ratio
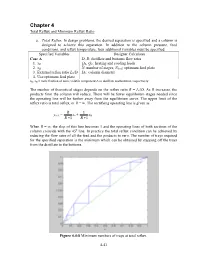
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

Members/Downloads/Docs/Distilling Instructions Super Reflux Condenser.Pdf
Distilling Instructions (Super Reflux Condenser) 9 Adams Road Palmerston NT 0830 Tel: (08) 8932 7700 Website: www.ozbrew.com Shop 17 Homemaker Village Millner NT Fax: (08) 8932 7622 Email: [email protected] Before reading these instructions, make sure you have read the information sheet called “Distilling Overview” which is also available in the downloads section of the Oz Brew website. The “Distilling Overview” sheet will provide useful information about the legality of stills and their usages, as well as outlining some good overall basics that will help provide essential information about this process. There are many types of stills, boilers, condensers & carbon filters available on the market. Different products and brands, may use different instructions. These instructions cover one of the most practical methods of distilling using a 25L boiler, a (super) reflux condenser and a Still Spirits Z- carbon filter, which we believe is one of the most effective value for money packages available. Read through all of your instructions including the Helpful Hints in each section before proceeding through the steps in each process. This will ensure you are aware of any tips that may make the process 25 Litre Boiling Pot Z Carbon Filter Super Reflux Condenser easier for you. (3 in 1 Fermenter) Typically produces a high yield of approximately 4-5L @ 80% - 90% V. Stage 1 - Sterilisation 1. Everything used in the fermentation process must be cleaned and sterilised before and after use. This includes the 3 in 1 fermenter, airlock and stirrer. 2. For optimum cleaning, we recommend Combo Cleaner Washing / Steriliser. Wash through all of your equipment with this solution and rinse thoroughly with cold tap water. -

A Smartphone Biosensor Based on Analysing Structural Colour of Porous Silicon† Cite This: Analyst, 2019, 144, 3942 Tengfei Cao, a Yiliang Zhao, a Crystal A
Analyst View Article Online PAPER View Journal | View Issue A smartphone biosensor based on analysing structural colour of porous silicon† Cite this: Analyst, 2019, 144, 3942 Tengfei Cao, a Yiliang Zhao, a Crystal A. Nattoo, b,c Rabeb Layouni d and Sharon M. Weiss *a,c We report a smartphone compatible, low-cost porous silicon biosensor, which correlates the structural colour of a porous silicon microcavity (PSiM) to spectral peak position. Molecules captured in the PSiM cause a colour change that can be quantified through image analysis. Minimal external accessories are employed. Spectrometer measurements of the PSiM reflectance spectrum shifts are carried out concur- rently with the smartphone measurements to benchmark the accuracy of the smartphone biosensor. We Received 4th January 2019, estimate that the smartphone biosensor supports an equivalent accuracy of 0.33 nm for the detection of Accepted 5th May 2019 colour changes corresponding to spectral shifts of the PSiM. Biosensing functionality is demonstrated DOI: 10.1039/c9an00022d using a biotin–streptavidin assay with an estimated detection limit of 500 nM. The PSiM-smartphone bio- rsc.li/analyst sensor is a promising platform for label-free point of care diagnostics. 1. Introduction designed for a single purpose instead of multi-parameter testing. Moreover, the data processing capabilities of these Point-of-care (POC) testing is a fast-growing sector in the sensors are usually basic and not intended for more compli- diagnostics market due to the convenience and low cost of cated detection and analysis tasks. Over the past few years, POC measurements.1 Biosensors are the crucial elements of smartphones have emerged as a promising host platform for POC diagnostics, which transform biomolecular or medical POC diagnostics systems due to their ubiquitous availability information into easily understandable readouts.