Building a Home Distillation Apparatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of the Enzymatic Mechanism of Nitroglycerin Bioactivation
Identification of the enzymatic mechanism of nitroglycerin bioactivation Zhiqiang Chen*†, Jian Zhang†, and Jonathan S. Stamler*†‡§ *Howard Hughes Medical Institute, Departments of †Medicine and §Biochemistry, Duke University Medical Center, Durham, NC 27710 Communicated by Irwin Fridovich, Duke University Medical Center, Durham, NC, April 15, 2002 (received for review March 26, 2002) Nitroglycerin (glyceryl trinitrate, GTN), originally manufactured by synthesized according to ref. 17 and purified by TLC. 1,2-GDN Alfred Nobel, has been used to treat angina and heart failure for and 1,3-GDN was prepared by hydrolysis of GTN and purified over 130 years. However, the molecular mechanism of GTN bio- by TLC (18). [2-14C] GTN (55 mCi͞mmol) was obtained from transformation has remained a mystery and it is not well under- American Radiolabeled Chemicals (St. Louis). Disulfiram, chlo- stood why ‘‘tolerance’’ (i.e., loss of clinical efficacy) manifests over ral hydrate, cyanamide, acetaldehyde, phenylephrine, sodium time. Here we purify a nitrate reductase that specifically catalyzes nitroprusside (SNP), Q-Sepharose, DEAE-cellulose, and butyl- the formation of 1,2-glyceryl dinitrate and nitrite from GTN, lead- Sepharose were obtained from Sigma. Hydroxyapatite and ing to production of cGMP and relaxation of vascular smooth protease inhibitor (mixture set III) were from CalBiochem. muscle both in vitro and in vivo, and we identify it as mitochondrial The cGMP assay (125I) kit was purchased from Amersham aldehyde dehydrogenase (mtALDH). We also show that mtALDH is Pharmacia. inhibited in blood vessels made tolerant by GTN. These results demonstrate that the biotransformation of GTN occurs predomi- Enzyme Purification. Mouse RAW264.7 cells (50-g pellet; Ϸ1010 nantly in mitochondria through a novel reductase action of cells) were disrupted by sonication in 30 mM phosphate buffer mtALDH and suggest that nitrite is an obligate intermediate in (KPi), pH 7.5 containing 1 mM DTT, 0.5 mM EDTA, and generation of NO bioactivity. -

Innovation in Continuous Rectification for Tequila Production
processes Communication Innovation in Continuous Rectification for Tequila Production Estarrón-Espinosa Mirna, Ruperto-Pérez Mariela, Padilla-de la Rosa José Daniel * and Prado-Ramírez Rogelio * Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Av. Normalistas No. 800, C.P. 44720 Guadalajara, Jalisco, Mexico; [email protected] (E.-E.M.); [email protected] (R.-P.M.) * Correspondence: [email protected] (P.-d.l.R.J.D.); [email protected] (P.-R.R.); Tel.: +33-33455200 (P.-d.l.R.J.D.) Received: 23 March 2019; Accepted: 6 May 2019; Published: 14 May 2019 Abstract: In this study, a new process of continuous horizontal distillation at a pilot level is presented. It was applied for the first time to the rectification of an ordinario fraction obtained industrially. Continuous horizontal distillation is a new process whose design combines the benefits of both distillation columns, in terms of productivity and energy savings (50%), and distillation stills in batch, in terms of the aromatic complexity of the distillate obtained. The horizontal process of continuous distillation was carried out at the pilot level in a manual mode, obtaining five accumulated fractions of distillate that were characterized by gas chromatography (GC-FID). The tequila obtained from the rectification process in this new continuous horizontal distillation process complies with the content of methanol and higher alcohols regulated by the Official Mexican Standard (NOM-006-SCFI-2012). Continuous horizontal distillation of tequila has potential energy savings of 50% compared to the traditional process, besides allowing products with major volatile profiles within the maximum limits established by the regulation for this beverage to be obtained. -

Page2 Sidebar 1.Pdf
Headquarters & Forge Americas Office [email protected] [email protected] +49 (0) 7161 / 97830 215.242.6806 +49 (0) 7161 / 978321 fax fax 215.701.9725 artisan distilling systems 600 liter whiskey still the fine art of distillery technology Germany’s oldest distillery fabricator, since 1869, combining traditional family craftsmanship with leading eau-de-vie distillery innovations and technologies. Meticulously custom-crafted artisan copper pot still systems for all the great distilling traditions, and efficient continuous plants, grappa distillery in copper and stainless steel, for all capacities and applications: 450 liter artisan pot stills – brandy & vodka vodka, whiskey, eaux-de-vie, brandy, rum, gin, grappa, tequila, aguardientes… 1000 liter artisan vodka system 650 liter system with CADi automation continuous mash stripping column C. CARL Ziegelstraße 21 Americas Office Brewing & Distilling Ing. GmbH D-73033 Göppingen PO Box 4388 Technologies Corp. www.christiancarl.com Germany Philadelphia, PA 19118-8388 www.brewing-distilling.com CARL artisan distillery systems the fine art of distillery technology CARL custom-builds each distillery to order in our family shop near Stuttgart in Swabia, with the attention and care of crafting a finely- tuned instrument. All-the-while, we stay focused on the continued development of our distillery technology. There are always new ideas and realizations, such our the in-house developed CARL CADi distillery automation or our patented aroma bubble plate technologies, Our innovations have fostered CARL’s nearly 140 years of family tradition and experience as Germany’s oldest and most respected distillery fabricator, with thousands of successful commissions worldwide. form and function Our diverse customers, from small farmers to winemakers to brewers to large spirits houses, show great enthusiasm and appreciation for the aesthetics and functionality of a CARL distillery: its design, its form, classic and intuitively easy to understand, clear in conception. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

Modelling of Crude Oil Distillation
Modelling of Crude Oil Distillation Modellering av råoljedestillation JENN Y BEATRIC E SOUC K K T H C h em i ca l S ci en c e and E n gi n e e r i n g Master’s Thesis in Chemical Engineering Stockholm, Sweden 2012 " One never notices what has been done; one can only see what remains to be done" (Marie Curie, 1867-1934) 1 Sammanfattning Under de föhållanden som reservoarens miljö erbjuder, definieras en petroleumvätska av dess termodynamiska och volymetriska egenskaper och av dess fysikalisk-kemiska egenskaper. För att korrekt simulera bearbetningen av dessa vätskor under produktion, deras beteende modelleras från experimentella data Med tillkomsten av nya regler och oflexibilitet som finns på tullbestämmelser vid gränserna idag, har forskningscenter stora svårigheter att få större mängder prover levererade. Av den anledningen, trots att det finns flera metoder för att karakterisera de olika komponenterna av råolja, tvingas laboratorier att vända sig mer och mer till alternativa analysmetoder som kräver mindre provvolymer: mikrodestillation, gaskromatografi, etc. Mikrodestillation, som är en snabb och helt datoriserad teknik, visar sig kunna ersätta standarddestillation för analys av flytande petroleumprodukter. Fördelar med metoden jämfört med standarddestillering är minskad arbetstidsåtgång med minst en faktor 4. Därtill krävs endast en [24] begränsad provvolym (några mikroliter) i jämförelse med standarddestillation. Denna rapport syftar till att skapa en enkel modell som kan förutsäga avkastningskurvan av fysisk destillation, utan att använda mikrodestillationsteknik. De resultat som erhölls genom gaskromatografiska analyser möjliggjorde modelleringen av det vätskebeteendet hos det analyserade provet. Efter att ha identifierat och behandlat praktiskt taget alla viktiga aspekter av mikro destillation genom simuleringar med PRO/II, fann jag att, oberoende av inställningen och den termodynamiska metod som används, det alltid finns stora skillnader mellan simulering och mikro destillation. -

Alchemist's Handbook-First Edition 1960 from One to Ten
BY THE SAME AUTHOR wqt Drei NoveIlen (German) 1932 The Alchemist's Handbook-First Edition 1960 From One to Ten . .. .. 1966 Alrqtuttaf!i Praxis Spagyrica Philosophica 1966 The Seven Rays of the Q.B.L.-First Edition 1968 Praetische Alchemie irn Zwanzigsten Jahrundert 1970 ~aubhnnk (Practical Alchemy in the 20th Century-German) Der Mensch und die kosmischen Zyklen (German) 1971 (Manual for Practical Laboratory Alchemy) Men and the Cycles of the Universe 1971 Von Eins bis Zehn (From One to Ten-German) 1972 El Hombre y los Ciclos del Universo (Spanish) 1972 by Die Sieben Strahlen der Q.B.L. 1973 (The Seven Rays of the Q.B.L.-German) FRATER ALBERTUS SAMUEL WEISER New York CONTENTS Foreword 6 Preface to the First Edition 10 Preface to the Second Revised Edition 13 Chapter I Introduction to Alchemy 14 Samuel Weiser, Inc. Chapter 11 740 Broadway The Lesser Circulation 24 New York, N.Y. 10003 Chapter III First Published 1960 The Herbal Elixir Revised Edition 1974 Chapter IV Third Printing 1978 Medicinal Uses 43 Chapter V © 1974 Paracelsus Research Society Herbs and Stars 47 Salt Lake City, Utah, U.S.A. Chapter VI Symbols in Alchemy 56 ISBN 0 87728 181 5 Chapter VII Wisdom of the Sages 65 Conclusion 100 Alchemical Manifesto 120 ILLUSTRATIONS On the Way to the Temple 5 Soxhlet Extractor 34 Basement Laboratory 41 Essential Equipment 42 Printed in U.S.A. by Qabalistic Tree of Life 57 NOBLE OFFSET PRINTERS, INC. NEW YORK, N.Y. 10003 Alchemical Signs 58 ORIGINAL OIL PAINTING AT PARACELSUS RESEARCH SOCIETY .. -

Addendum CSS 2021
Addendum regarding: The 2021 Certified Specialist of Spirits Study Guide, as published by the Society of Wine Educators Note: This document outlines the substantive changes to the 2021 Study Guide as compared to the 2020 version of the CSW Study Guide. All page numbers reference the 2020 version. Please note that in addition to the information noted below, the tables concerning top-selling brands of particular types of spirits have been updated to reflect the most current statistics available. Page 10: The information regarding congeners was expanded to include the following: Congeners: The preceding explanation of distillation has been simplified by using the example of a solution made of only ethyl alcohol and water. However, many other compounds are created during fermentation and as a result, there are other compounds besides water and alcohol present in a fermented solution. Known as congeners, these compounds are responsible for much of the aroma and flavor—besides that of pure ethyl alcohol and water—of a fermented beverage or a distilled spirit. Specific congeners include the various acids, esters, aldehydes, fusel oils, and alcohols (other than ethanol) that are developed during fermentation. During distillation, congeners may vaporize and blend in with the ethanol–water vapors; however, each specific congener will react differently based on three factors: boiling point, solubility (in ethanol and water), and specific gravity. In addition, the heat of the distillation process—via a series of chemical changes known as pyrolysis—may cause some compounds to change form, creating new and different congeners that may be passed onto the finished product. -

Tailoring Diesel Bioblendstock from Integrated Catalytic Upgrading of Carboxylic Acids: a “Fuel Property First” Approach Xiangchen Huoa,B, Nabila A
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2019 Tailoring Diesel Bioblendstock from Integrated Catalytic Upgrading of Carboxylic Acids: A “Fuel Property First” Approach Xiangchen Huoa,b, Nabila A. Huqa, Jim Stunkela, Nicholas S. Clevelanda, Anne K. Staracea, Amy E. Settlea,b, Allyson M. Yorka,b, Robert S. Nelsona, David G. Brandnera, Lisa Foutsa, Peter C. St. Johna, Earl D. Christensena, Jon Lueckea, J. Hunter Mackc, Charles S. McEnallyd, Patrick A. Cherryd, Lisa D. Pfefferled, Timothy J. Strathmannb, Davinia Salvachúaa, Seonah Kima, Robert L. McCormicka, Gregg T. Beckhama, Derek R. Vardona* aNational Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, CO, United States bColorado School of Mines, 1500 Illinois St., Golden, CO, United States cUniversity of Massachusetts Lowell, 220 Pawtucket St., Lowell, MA, United States dYale University, 9 Hillhouse Avenue, New Haven, CT, United States *[email protected] Section S1: Experimental Methods Catalyst synthesis and characterization Pt/Al2O3 catalyst was prepared by strong electrostatic adsorption method with chloroplatinic acid hexahydrate as Pt precursor. Al2O3 of 30-50 mesh and Pt precursor were added to deionized water, and solution pH was adjusted to 3 by adding HCl. After stirring overnight, the catalyst particles were recovered by filtration extensively washed with -1 deionized water. The catalyst was dried in the air and reduced in flowing H2 (200 mL min ) at 300°C for 4 h. BET surface area was determined by nitrogen physisorption using a Quadrasorb SI™ surface area analyzer from Quantachrome Instruments. Samples of ~80–120 mg were measured using a 55-point nitrogen adsorption/desorption curve at -196°C. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -
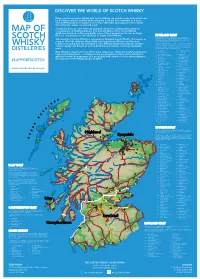
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character.