Chemical Laboratory Techniques - Gelosa D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Asphalt Pavement – Micro-Sampling and Micro-Extraction Methods
Technical White Paper Asphalt Pavement – Micro-sampling and Micro-extraction Methods Fundamental Properties of Asphalts and Modified Asphalts III Product: FP 09 March 2015 Prepared for Federal Highway Administration Contract No. DTFH61-07-D-00005 By Michael J. Farrar, R. William Grimes, Scott Wiseman, and Jean-Pascal Planche Western Research Institute 3474 North 3rd Street Laramie, WY 82072 www.westernresearch.org TABLE OF CONTENTS INTRODUCTION..........................................................................................................................1 Background ................................................................................................................................1 Definition of Micro-sampling and Micro-extraction .................................................................2 Chemical and Rheological Characterization of Micro-extracted Asphalt .................................3 EXPERIMENTAL .........................................................................................................................3 RESULTS AND DISCUSSION ....................................................................................................3 Micro-sampling ..........................................................................................................................3 First Experiment (oxidation during drilling)..............................................................................6 Second Experiment (oxidation during drilling) .........................................................................8 -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -

Immersion Cooling of Electronics in Dod Installations
LBNL-1005666 Immersion Cooling of Electronics in DoD Installations Henry Coles and Magnus Herrlin Energy Technologies Area May 2016 DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or The Regents of the University of California. ABSTRACT A considerable amount of energy is consumed to cool electronic equipment in data centers. A method for substantially reducing the energy needed for this cooling was demonstrated. The method involves immersing electronic equipment in a non-conductive liquid that changes phase from a liquid to a gas. The liquid used was 3M Novec 649. Two-phase immersion cooling using this liquid is not viable at this time. The primary obstacles are IT equipment failures and costs. However, the demonstrated technology met the performance objectives for energy efficiency and greenhouse gas reduction. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -
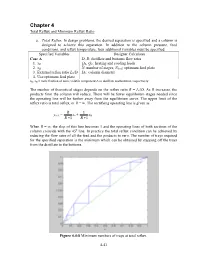
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

Environmental Health and Safety Vacuum Traps
Environmental Health and Safety Vacuum Traps Always place an appropriate trap between experimental apparatus and the vacuum source. The vacuum trap: • protects the pump, pump oil and piping from the potentially damaging effects of the material; • protects people who must work on the vacuum lines or system, and; • prevents vapors and related odors from being emitted back into the laboratory or system exhaust. Improper trapping can allow vapor to be emitted from the exhaust of the vacuum system, resulting in either reentry into the laboratory and building or potential exposure to maintenance workers. Proper traps are important for both local pumps and building systems. Proper Trapping Techniques To prevent contamination, all lines leading from experimental apparatus to the vacuum source must be equipped with filtration or other trapping as appropriate. • Particulates: use filtration capable of efficiently trapping the particles in the size range being generated. • Biological Material: use a High Efficiency Particulate Air (HEPA) filter. Liquid disinfectant (e.g. bleach or other appropriate material) traps may also be required. • Aqueous or non-volatile liquids: a filter flask at room temperature is adequate to prevent liquids from getting to the vacuum source. • Solvents and other volatile liquids: use a cold trap of sufficient size and cold enough to condense vapors generated, followed by a filter flask capable of collecting fluid that could be aspirated out of the cold trap. • Highly reactive, corrosive or toxic gases: use a sorbent canister or scrubbing device capable of trapping the gas. Environmental Health and Safety 632-6410 January 2010 EHSD0365 (01/10) Page 1 of 2 www.stonybrook.edu/ehs Cold Traps For most volatile liquids, a cold trap using a slush of dry ice and either isopropanol or ethanol is sufficient (to -78 deg. -

A Sample AMS Latex File
Li, L. et al. (2021): JoSS, Vol. 10, No. 1, pp. 983–993 (Peer-reviewed article available at www.jossonline.com) www.adeepakpublishing.com www. JoSSonline.com Preliminary Thermal Validation Tests for Education-Class CubeSats and Weather- Balloon Payloads Lingqi Li and Kenjiro S. Lay Penn State University State College, PA, US Masataka Okutsu Penn State University Abington Abington, PA, US Abstract Low development and launch costs of CubeSats, a type of small spacecraft typically one to three liters in volume, have made space science accessible to educational institutions, offering engaging opportunities for stu- dents in the science, technology, engineering, and mathematics (STEM) disciplines. Some university teams work- ing on these education-class CubeSats conduct high-altitude flight experiments using balloons to test their instru- ments in the harsh environment at the edges of the troposphere and the stratosphere. Whether for the balloon experiment or for the actual spaceflight, temperatures of the operating environments are of concern. Instruments flown in space must be qualified for wide thermal ranges (e.g., −40°C to 70°C) in vacuum conditions. Likewise, instruments flown on the balloons must be able to operate in a similarly large range of temperatures (e.g., −50°C to 50°C) in the reduced pressure environment. Unfortunately, a thermal-vacuum chamber—standard testing equipment for spacecraft—is not accessible to many university teams. This paper presents incubator testing and cooling-bath testing methods as preliminary thermal validation tests that may be carried out easily, safely, and inexpensively, without any need for the expensive thermal-vacuum chamber. We also discuss an add-on demon- stration in which a CubeSat prototype was flown on a weather balloon to an altitude of ~16 km. -

Members/Downloads/Docs/Distilling Instructions Super Reflux Condenser.Pdf
Distilling Instructions (Super Reflux Condenser) 9 Adams Road Palmerston NT 0830 Tel: (08) 8932 7700 Website: www.ozbrew.com Shop 17 Homemaker Village Millner NT Fax: (08) 8932 7622 Email: [email protected] Before reading these instructions, make sure you have read the information sheet called “Distilling Overview” which is also available in the downloads section of the Oz Brew website. The “Distilling Overview” sheet will provide useful information about the legality of stills and their usages, as well as outlining some good overall basics that will help provide essential information about this process. There are many types of stills, boilers, condensers & carbon filters available on the market. Different products and brands, may use different instructions. These instructions cover one of the most practical methods of distilling using a 25L boiler, a (super) reflux condenser and a Still Spirits Z- carbon filter, which we believe is one of the most effective value for money packages available. Read through all of your instructions including the Helpful Hints in each section before proceeding through the steps in each process. This will ensure you are aware of any tips that may make the process 25 Litre Boiling Pot Z Carbon Filter Super Reflux Condenser easier for you. (3 in 1 Fermenter) Typically produces a high yield of approximately 4-5L @ 80% - 90% V. Stage 1 - Sterilisation 1. Everything used in the fermentation process must be cleaned and sterilised before and after use. This includes the 3 in 1 fermenter, airlock and stirrer. 2. For optimum cleaning, we recommend Combo Cleaner Washing / Steriliser. Wash through all of your equipment with this solution and rinse thoroughly with cold tap water.