Comparison of Ceftriaxone and Cefazolin Sodium Antibiotic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and Its Application
Infect Dis Ther (2017) 6:57–67 DOI 10.1007/s40121-016-0144-8 REVIEW Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and its Application Juwon Yim . Leah M. Molloy . Jason G. Newland Received: November 10, 2016 / Published online: December 30, 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com ABSTRACT infections, CABP caused by penicillin- and ceftriaxone-resistant S. pneumoniae and Ceftaroline is a novel cephalosporin recently resistant Gram-positive infections that fail approved in children for treatment of acute first-line antimicrobial agents. However, bacterial skin and soft tissue infections and limited data are available on tolerability in community-acquired bacterial pneumonia neonates and infants younger than 2 months (CABP) caused by methicillin-resistant of age, and on pharmacokinetic characteristics Staphylococcus aureus, Streptococcus pneumoniae in children with chronic medical conditions and other susceptible bacteria. With a favorable and those with invasive, complicated tolerability profile and efficacy proven in infections. In this review, the microbiological pediatric patients and excellent in vitro profile of ceftaroline, its mechanism of action, activity against resistant Gram-positive and and pharmacokinetic profile will be presented. Gram-negative bacteria, ceftaroline may serve Additionally, clinical evidence for use in as a therapeutic option for polymicrobial pediatric patients and proposed place in therapy is discussed. Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/ 1F47F0601BB3F2DD. Keywords: Antibiotic resistance; Ceftaroline J. Yim (&) fosamil; Children; Methicillin-resistant St. John Hospital and Medical Center, Detroit, MI, Staphylococcus aureus; Streptococcus pneumoniae USA e-mail: [email protected] L. -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -
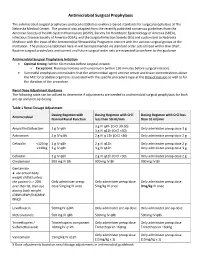
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Combination Therapy in Complicated Infections Due to S. Aureus
Combination therapy in complicated infections due to S. aureus Alex Soriano ([email protected]) Service of Infectious Diseases Hospital Clínic of Barcelona ESCMIDUniversity of Barcelona eLibrary IDIBAPS © by author Staphylococcal dissemination to different organs 30 min after i.v. Infection (using a real-time Sureward, et al. imaging) Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary Liver, Kupfer cell (purple ) © byS. aureus author(green) Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 eradication Large cluster of S. from blood aureus inside of KC. Located in phagolysosomes ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary Vancosomes: vancomycin vancomycin (1h before ©or after by) authorwithin liposomes Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 Animal model of S aureus bacteremia. Treatment started after 24h of the infection ESCMID eLibrary single dose © by author ESCMID CaseeLibrary #1 © by author Medical history: A 27 y-o man, without co-morbidity. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

Staphylococcus Aureus Bloodstream Infection Treatment Guideline
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI). The recommendations below are guidelines for care and are not meant to replace clinical judgment. The initial page includes a brief version of the guidance followed by a more detailed discussion of the recommendations with supporting evidence. Also included is an algorithm describing management of patients with blood cultures positive for gram-positive cocci. Brief Key Points: 1. Don’t ignore it – Staphylococcus aureus isolated from a blood culture is never a contaminant. All patients with S. aureus in their blood should be treated with appropriate antibiotics and evaluated for a source of infection. 2. Control the source a. Removing infected catheters and prosthetic devices – Retention of infected central venous catheters and prosthetic devices in the setting of S. aureus bacteremia (SAB) has been associated with prolonged bacteremia, treatment failure and death. These should be removed if medically possible. i. Retention of prosthetic material is associated with an increased likelihood of SAB relapse and removal should be considered even if not clearly infected b. Evaluate for metastatic infections (endocarditis, osteomyelitis, abscesses, etc.) – Metastatic infections and endocarditis are quite common in SAB (11-31% patients with SAB have endocarditis). i. All patients should have a thorough history taken and exam performed with any new complaint evaluated for possible metastatic infection. ii. Echocardiograms should be strongly considered for all patients with SAB iii. All patients with a prosthetic valve, pacemaker/ICD present, or persistent bacteremia (follow up blood cultures positive) should undergo a transesophageal echocardiogram 3. -

Safety and Efficacy of Ceftaroline Fosamil in the Management of Community-Acquired Bacterial Pneumonia Heather F
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Scholarly Papers 2014 Safety and Efficacy of Ceftaroline Fosamil in the Management of Community-Acquired Bacterial Pneumonia Heather F. DeBellis Kimberly L. Barefield Philadelphia College of Osteopathic Medicine, [email protected] Follow this and additional works at: https://digitalcommons.pcom.edu/scholarly_papers Part of the Medicine and Health Sciences Commons Recommended Citation DeBellis, Heather F. and Barefield, Kimberly L., "Safety and Efficacy of Ceftaroline Fosamil in the Management of Community- Acquired Bacterial Pneumonia" (2014). PCOM Scholarly Papers. 1913. https://digitalcommons.pcom.edu/scholarly_papers/1913 This Article is brought to you for free and open access by DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Scholarly Papers by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Open Access: Full open access to Clinical Medicine Reviews this and thousands of other papers at http://www.la-press.com. in Therapeutics Safety and Efficacy of Ceftaroline Fosamil in the Management of Community- Acquired Bacterial Pneumonia Heather F. DeBellis and Kimberly L. Tackett South University School of Pharmacy, Savannah, GA, USA. ABSTR ACT: Ceftaroline fosamil is a new fifth-generation cephalosporin indicated for the treatment of community-acquired bacterial pneumonia (CABP). It possesses antimicrobial effects against both Gram-positive and Gram-negative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), but not against anaerobes. Organisms covered by this novel agent that are commonly associated with CABP are Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, and Klebsiella pneumoniae; however, ceftaroline fosamil lacks antimicrobial activity against Pseudomonas and Acinetobacter species. -

Management of Penicillin and Beta-Lactam Allergy
Management of Penicillin and Beta-Lactam Allergy (NB Provincial Health Authorities Anti-Infective Stewardship Committee, September 2017) Key Points • Beta-lactams are generally safe; allergic and adverse drug reactions are over diagnosed and over reported • Nonpruritic, nonurticarial rashes occur in up to 10% of patients receiving penicillins. These rashes are usually not allergic and are not a contraindication to the use of a different beta-lactam • The frequently cited risk of 8 to 10% cross-reactivity between penicillins and cephalosporins is an overestimate based on studies from the 1970’s that are now considered flawed • Expect new intolerances (i.e. any allergy or adverse reaction reported in a drug allergy field) to be reported after 0.5 to 4% of all antimicrobial courses depending on the gender and specific antimicrobial. Expect a higher incidence of new intolerances in patients with three or more prior medication intolerances1 • For type-1 immediate hypersensitivity reactions (IgE-mediated), cross-reactivity among penicillins (table 1) is expected due to similar core structure and/or major/minor antigenic determinants, use not recommended without desensitization • For type-1 immediate hypersensitivity reactions, cross-reactivity between penicillins (table 1) and cephalosporins is due to similarities in the side chains; risk of cross-reactivity will only be significant between penicillins and cephalosporins with similar side chains • Only type-1 immediate hypersensitivity to a penicillin manifesting as anaphylaxis, bronchospasm, -

Cephalosporins Can Be Prescribed Safely for Penicillin-Allergic Patients ▲
JFP_0206_AE_Pichichero.Final 1/23/06 1:26 PM Page 106 APPLIED EVIDENCE New research findings that are changing clinical practice Michael E. Pichichero, MD University of Rochester Cephalosporins can be Medical Center, Rochester, NY prescribed safely for penicillin-allergic patients Practice recommendations an allergic reaction to cephalosporins, ■ The widely quoted cross-allergy risk compared with the incidence of a primary of 10% between penicillin and (and unrelated) cephalosporin allergy. cephalosporins is a myth (A). Most people produce IgG and IgM antibodies in response to exposure to ■ Cephalothin, cephalexin, cefadroxil, penicillin1 that may cross-react with and cefazolin confer an increased risk cephalosporin antigens.2 The presence of of allergic reaction among patients these antibodies does not predict allergic, with penicillin allergy (B). IgE cross-sensitivity to a cephalosporin. ■ Cefprozil, cefuroxime, cefpodoxime, Even penicillin skin testing is generally not ceftazidime, and ceftriaxone do not predictive of cephalosporin allergy.3 increase risk of an allergic reaction (B). Reliably predicting cross-reactivity ndoubtedly you have patients who A comprehensive review of the evidence say they are allergic to penicillin shows that the attributable risk of a cross- U but have difficulty recalling details reactive allergic reaction varies and is of the reactions they experienced. To be strongest when the chemical side chain of safe, we often label these patients as peni- the specific cephalosporin is similar to that cillin-allergic without further questioning of penicillin or amoxicillin. and withhold not only penicillins but Administration of cephalothin, cepha- cephalosporins due to concerns about lexin, cefadroxil, and cefazolin in penicillin- potential cross-reactivity and resultant IgE- allergic patients is associated with a mediated, type I reactions. -

Antibiotic Prophylaxis 2017 Update
Distribution Information AAE members may reprint this position statement for distribution to patients or referring dentists. Antibiotic Prophylaxis 2017 Update AAE Quick Reference Guide About This Document This paper is designed to Endocarditis Prophylaxis Recommendations provide scientifically based These recommendations are taken from 2017 American Heart Association and guidance to clinicians American College of Cardiology focused update of the 2014 AHA/ADA Guideline regarding the use of antibiotics for Management of Patients with Valvular Disease (1) and cited by the ADA (2). in endodontic treatment. Thank you to the Special Committee on Antibiotic Use in Prophylaxis against infective endocarditis is reasonable before dental Endodontics: Ashraf F. Fouad, procedures that involve manipulation of gingival tissue, manipulation of the Chair, B. Ellen Byrne, Anibal R. periapical region of teeth, or perforation of the oral mucosa in patients with the Diogenes, Christine M. Sedgley following: and Bruce Y. Cha. In 2017, the AHA and American College of Cardiology (ACC) published ©2017 a focused update (5) to their previous guidelines on the management of valvular heart disease. This reinforced their previous recommendations that AP is reasonable for the subset of patients at increased risk of developing IE and at high risk of experiencing adverse outcomes from IE (5). Their key recommendations were: 1. Prosthetic cardiac valves, including transcatheter-implanted prostheses and homografts. 2. Prosthetic material used for cardiac valve repair, such as annuloplasty rings and chords. 3. Previous IE. 4. Unrepaired cyanotic congenital heart disease or repaired congenital heart disease, with residual shunts or valvular regurgitation at the site of or adjacent to the site of a prosthetic patch or prosthetic device. -

A Thesis Entitled an Oral Dosage Form of Ceftriaxone Sodium Using Enteric
A Thesis entitled An oral dosage form of ceftriaxone sodium using enteric coated sustained release calcium alginate beads by Darshan Lalwani Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences with Industrial Pharmacy Option _________________________________________ Jerry Nesamony, Ph.D., Committee Chair _________________________________________ Sai Hanuman Sagar Boddu, Ph.D, Committee Member _________________________________________ Youssef Sari, Ph.D., Committee Member _________________________________________ Patricia R. Komuniecki, PhD, Dean College of Graduate Studies The University of Toledo May 2015 Copyright 2015, Darshan Narendra Lalwani This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of An oral dosage form of ceftriaxone sodium using enteric coated sustained release calcium alginate beads by Darshan Lalwani Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences with Industrial Pharmacy option The University of Toledo May 2015 Purpose: Ceftriaxone (CTZ) is a broad spectrum semisynthetic, third generation cephalosporin antibiotic. It is an acid labile drug belonging to class III of biopharmaceutical classification system (BCS). It can be solvated quickly but suffers from the drawback of poor oral bioavailability owing to its limited permeability through -

Cefazolin for Injection, Usp
CEFAZOLIN- cefazolin injection, powder, for solution West-Ward Pharmaceuticals Corp ---------- CEFAZOLIN FOR INJECTION, USP Rx ONLY PHARMACY BULK PACKAGE - NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefazolin for injection and other antibacterial drugs, cefazolin for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Cefazolin for Injection, USP is a semi-synthetic cephalosporin for parenteral administration. It is the sodium salt of (6R, 7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)]thio]methyl]-8-oxo-7-[2(1H-tetrazol-1- yl)acetoamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. The structural formula is as follows: Cefazolin for Injection, USP is a white to cream sterile powder. The color of Cefazolin for Injection, USP solutions may range from pale yellow to yellow without a change in potency. The pH ranges from 4.0 and 6.0 for a solution containing 100 mg of cefazolin per mL. Cefazolin for Injection, USP is supplied in 10 or 20 grams Pharmacy Bulk Packages. Each Pharmacy Bulk Package contains Cefazolin Sodium, USP equivalent to 10 or 20 grams of cefazolin. The sodium content is approximately 48 mg (2.1 mEq) per gram of cefazolin sodium. It is to be administered by intravenous route. A Pharmacy Bulk Package is a container of a sterile preparation for intravenous use that contains many single doses. The contents are intended for use in a pharmacy admixture service and are restricted to the preparation of admixtures for intravenous infusion.