Staphylococcus Aureus Bloodstream Infection Treatment Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C-Reactive Protein and Albumin Kinetics Before Community-Acquired Bloodstream Infections – Cambridge.Org/Hyg a Danish Population-Based Cohort Study
Epidemiology and Infection C-reactive protein and albumin kinetics before community-acquired bloodstream infections – cambridge.org/hyg a Danish population-based cohort study 1 1,2,3 1 4 5 Original Paper O. S. Garvik , P. Póvoa , B. Magnussen , P. J. Vinholt , C. Pedersen , T. G. Jensen6, H. J. Kolmos6, A. T. Lassen7 and K. O. Gradel1 Cite this article: Garvik OS, Póvoa P, Magnussen B, Vinholt PJ, Pedersen C, Jensen 1Research Unit of Clinical Epidemiology, Institute of Clinical Research, University of Southern Denmark, and Center TG, Kolmos HJ, Lassen AT, Gradel KO (2020). for Clinical Epidemiology, Odense University Hospital, Kløvervænget 30, Entrance 216, ground floor, 5000 Odense C-reactive protein and albumin kinetics before 2 community-acquired bloodstream infections – C, Denmark; NOVA Medical School, New University of Lisbon, Campo Mártires da Pátria 130, 1169-056 Lisbon, 3 a Danish population-based cohort study. Portugal; Polyvalent Intensive Care Unit, São Francisco Xavier Hospital, CHLO, Estrada do Forte do Alto do Duque, 4 Epidemiology and Infection 148,e38,1–6. 1449-005 Lisbon, Portugal; Department of Clinical Biochemistry and Pharmacology, Odense University Hospital, https://doi.org/10.1017/S0950268820000291 Sdr. Boulevard 29, entrance 40, 5000 Odense C, Denmark; 5Department of Infectious Diseases, Odense University Hospital, Sdr. Boulevard 29, entrance 20, 5000 Odense C, Denmark; 6Department of Clinical Microbiology, Odense Received: 30 October 2019 University Hospital, J.B. Winsløws Vej 21, 2nd floor, 5000 Odense C, Denmark and 7Department of Emergency Revised: 16 January 2020 Medicine, Odense University Hospital, Kløvervænget 25, entrance 63-65, 5000 Odense C, Denmark Accepted: 22 January 2020 Key words: Abstract Albumin; C-reactive protein; community acquired bloodstream infections Early changes in biomarker levels probably occur before bloodstream infection (BSI) is diag- nosed. -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200327 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number(s) 200327 Priority or Standard Standard Submit Date(s) December 29, 2009 Received Date(s) December 30, 2009 PDUFA Goal Date October 30, 2010 Division / Office Division of Anti-Infective and Ophthalmology Products Office of Antimicrobial Products Reviewer Name(s) Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD Review Completion October 29, 2010 Date Established Name Ceftaroline fosamil for injection (Proposed) Trade Name Teflaro Therapeutic Class Cephalosporin; ß-lactams Applicant Cerexa, Inc. Forest Laboratories, Inc. Formulation(s) 400 mg/vial and 600 mg/vial Intravenous Dosing Regimen 600 mg every 12 hours by IV infusion Indication(s) Acute Bacterial Skin and Skin Structure Infection (ABSSSI); Community-acquired Bacterial Pneumonia (CABP) Intended Population(s) Adults ≥ 18 years of age Template Version: March 6, 2009 Reference ID: 2857265 Clinical Review Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD NDA 200327: Teflaro (ceftaroline fosamil) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ......................................... 9 1.1 Recommendation on Regulatory Action ........................................................... 10 1.2 Risk Benefit Assessment.................................................................................. 10 1.3 Recommendations for Postmarketing Risk Evaluation and Mitigation Strategies ........................................................................................................................ -

Family Practice
THE JOURNAL OF FAMILY ONLINE EXCLUSIVE PRACTICE Paul K. Carlton, Jr, MD, Brown recluse spider bite? FACS The Texas A&M University Consider this uniquely Health Science Center College Station, Tex conservative treatment [email protected] An antihistamine and observation work as well— and often better—than more intensive therapies. Practice recommendations oped it in 4 phases, which I describe in • Be concerned about brown recluse this article. Not only does this conser- envenomation when a patient vative approach consistently heal con- reports intensifying localized firmed brown recluse bite wounds, but pain disproportionate to physical should a bite be mistakenly attributed findings after a “bite” (C). to the brown recluse (or one of its rela- tives in the Loxosceles genus of spider), • Prescribe an oral antihistamine there is no harm to the patient, nor any alone to control symptoms, even big expense. with a necrotic wound, and mark the IN THIS ARTICLE patient’s progress over 24 hours (C). z Spider bite z Is a brown recluse • If the patient improves dramatically, to blame? or MRSA? continue the antihistamine; with little Due to limited experience among the Page E6 or no improvement, consider giving an wider medical community in identifying antibiotic with the antihistamine (C). spider envenomation,1-4 bite recognition and selection of appropriate therapy can Strength of recommendation (SOR) be difficult. A Good-quality patient-oriented evidence Early findings can be confusing. B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented Brown recluse bites typically feel like a evidence, case series pin prick. -

BLOOD INFECTIONS INTRODUCTION TYPES Of
BLOOD INFECTIONS Please supplement and learn theory on the basis of the lecture, Mim’s book and supplementary materials before class!!! INTRODUCTION PRINCIPLE: UNDER NATURAL CONDITIONS BLOOD IS STERILE IMPORTANT TERMS: Nosocomial infection ……………………………………………………………………………………………………………….. Bacteremia ………………………………………………………………………………………………………………………………. Viremia …………………………………………………………………………………………………………………………………….. Fungemia ………………………………………………………………………………………………………………………………….. Sepsis …………………………………………………………………………………………………………………………………………. SIRS …………………………………………………………………………………………………………………………………………….. MODS …………………………………………………………………………………………………………………………………………. ARDS …………………………………………………………………………………………………………………………………………… DIC ………………………………………………………………………………………………………………………………………………. CRBI …………………………………………………………………………………………………………………………………………….. BSI ………………………………………………………………………………………………………………………………………………. TYPES of BACTEREMIA .------------------------------------ ---------------------------------- ---------------------------------- •Examples: •Examples: •Examples: •-------------------------------------- •------------------------------------- •------------------------------------ -------------------------------------- •-------------------------------------- •-------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -------------------------------------- -

Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and Its Application
Infect Dis Ther (2017) 6:57–67 DOI 10.1007/s40121-016-0144-8 REVIEW Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and its Application Juwon Yim . Leah M. Molloy . Jason G. Newland Received: November 10, 2016 / Published online: December 30, 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com ABSTRACT infections, CABP caused by penicillin- and ceftriaxone-resistant S. pneumoniae and Ceftaroline is a novel cephalosporin recently resistant Gram-positive infections that fail approved in children for treatment of acute first-line antimicrobial agents. However, bacterial skin and soft tissue infections and limited data are available on tolerability in community-acquired bacterial pneumonia neonates and infants younger than 2 months (CABP) caused by methicillin-resistant of age, and on pharmacokinetic characteristics Staphylococcus aureus, Streptococcus pneumoniae in children with chronic medical conditions and other susceptible bacteria. With a favorable and those with invasive, complicated tolerability profile and efficacy proven in infections. In this review, the microbiological pediatric patients and excellent in vitro profile of ceftaroline, its mechanism of action, activity against resistant Gram-positive and and pharmacokinetic profile will be presented. Gram-negative bacteria, ceftaroline may serve Additionally, clinical evidence for use in as a therapeutic option for polymicrobial pediatric patients and proposed place in therapy is discussed. Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/ 1F47F0601BB3F2DD. Keywords: Antibiotic resistance; Ceftaroline J. Yim (&) fosamil; Children; Methicillin-resistant St. John Hospital and Medical Center, Detroit, MI, Staphylococcus aureus; Streptococcus pneumoniae USA e-mail: [email protected] L. -

Charm II Antibiotic Analysis—Grain
Charm ii Antibiotic Analysis for Grain Products PROCeDURAL FLOWCHART + Binding Tracer Reagent Tablet Tablet Sample Charm ii 7600 analyzer Incubate START STOP Centrifuge Families DeteCteD = Aminoglycosides = Amphenicols/Chloramphenicol Resuspend = Beta-lactams = Macrolides = Sulfonamides C2Soft = Tetracyclines (optional) Count Results SAMPLE SIZe 50 to 100 g Computer Report SAMPLE PREPARATION Homogenize product in extraction solution for 60 seconds. Filter or centrifuge for 3 minutes. sample printout Test supernatant. Date = 08/23/10 preparation time Approximately 10-15 minutes, Time = 14:28:12 depending on the number of Operator = 1 samples. Time Counted = 60 Sample I.D. = 7764 ASSAY TIME Approximately 10 minutes, depending on drug family. Assay = Chloramphenicol CAPACITY 6 to 12 samples in assay, Lot# = ATBL 014 depending on drug family. Control Point = 2564 Sample (CPM) = 3676 Interpretation = Not Found Charm sciences, inc. 659 Andover Street, Lawrence, MA 01843, USA | Tel: +1.978.687.9200 | www.charm.com Charm ii Antibiotic Analysis for Grain Products Charm ii Kit Drug test sensitivity 1 (ppb) aminoglycosides (STIIHG) Streptomycin 2000 Dihydrostreptomycin 7500 Gentamicin 5000 aminoglycosides (GIIHG) Gentamicin 1000 Neomycin 500 Beta-lactams (PIIG) Penicillin-G 200 Amoxicillin 450 Ampicillin 400 Cephapirin 200 Ceftiofur 500 Cloxacillin 2500 Oxacillin 3750 Dicloxacillin 2500 Cefazolin 1500 Cefodroxil 1500 Cefotaxime 400 Cephalexin 1500 Cephradine 1500 Cefquinome 1000 Hetacillin 400 Nafcillin 3000 Penethamate 200 Piperacillin 1000 Ticarcillin 3500 Chloramphenicol Chloramphenicol 40 Florfenicol 160 & other amphenicols (CIIHG) Thiamphenicol 200 Chloramphenicol (AIIHG) Chloramphenicol 5 macrolides (EIIG) Erythromycin 1000 Tylosin 1000 Spiramycin 1000 Pirlimycin 2000 Tilmicosin 1000 Lincomycin 2500 sulfonamides (SMIIHG) Sulfamethazine 500 Sulfadimethoxine 200 Sulfathiazole 400 Sulfadiazine 200 tetracyclines (TIIHG) Tetracycline 100 Chlortetracycline 800 Oxytetracycline 800 1 Exceed 90% positive at a 95% confidence limit Charm sciences, inc. -

UNASYN® (Ampicillin Sodium/Sulbactam Sodium)
® UNASYN (ampicillin sodium/sulbactam sodium) To reduce the development of drug-resistant bacteria and maintain the effectiveness of UNASYN® and other antibacterial drugs, UNASYN should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION UNASYN is an injectable antibacterial combination consisting of the semisynthetic antibacterial ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration. Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate and has a molecular weight of 371.39. Its chemical formula is C16H18N3NaO4S. The structural formula is: COONa O CH 3 N CH O 3 NH S NH 2 1 Reference ID: 4053909 Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia- 1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22. The structural formula is: COONa CH 3 O N CH 3 S O O UNASYN, ampicillin sodium/sulbactam sodium parenteral combination, is available as a white to off-white dry powder for reconstitution. UNASYN dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. The pH of the solutions is between 8.0 and 10.0. -

What Is Staphylococcus Aureus (Staph)?
What is Staphylococcus aureus (staph)? Staphylococcus aureus, often referred to simply as "staph," are bacteria commonly carried on the skin or in the nose of healthy people. Approximately 25% to 30% of the population is colonized (when bacteria are present, but not causing an infection) in the nose with staph bacteria. Sometimes, staph can cause an infection. Staph bacteria are one of the most common causes of skin infections in the United States. Most of these skin infections are minor (such as pimples and boils) and can be treated without antibiotics (also known as antimicrobials or antibacterials). However, staph bacteria also can cause serious infections (such as surgical wound infections, bloodstream infections, and pneumonia). What is MRSA (methicillin-resistant Staphylococcus aureus)? Some staph bacteria are resistant to antibiotics. MRSA is a type of staph that is resistant to antibiotics called beta-lactams. Beta-lactam antibiotics include methicillin and other more common antibiotics such as oxacillin, penicillin and amoxicillin. While 25% to 30% of the population is colonized with staph, approximately 1% is colonized with MRSA. Who gets staph or MRSA infections? Staph infections, including MRSA, occur most frequently among persons in hospitals and healthcare facilities (such as nursing homes and dialysis centers) who have weakened immune systems. These healthcare-associated staph infections include surgical wound infections, urinary tract infections, bloodstream infections, and pneumonia. Are people who are positive for the human immune deficiency virus (HIV) at increased risk for MRSA? Should they be taking special precautions? People with weakened immune systems, which include some patients with HIV infection, may be at risk for more severe illness if they get infected with MRSA. -
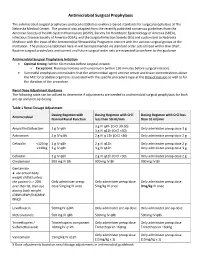
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®)
UnitedHealthcare® Commercial Medical Benefit Drug Policy Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®) Policy Number: 2021D0088F Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Community Plan Policy Coverage Rationale ....................................................................... 1 • Intravenous Iron Replacement Therapy (Feraheme®, Definitions ...................................................................................... 3 Injectafer®, & Monoferric®) Applicable Codes .......................................................................... 3 Background.................................................................................... 4 Benefit Considerations .................................................................. 4 Clinical Evidence ........................................................................... 5 U.S. Food and Drug Administration ............................................. 7 Centers for Medicare and Medicaid Services ............................. 8 References ..................................................................................... 8 Policy History/Revision Information ............................................. 9 Instructions for Use ....................................................................... 9 Coverage Rationale See Benefit Considerations This policy refers to the following intravenous iron replacements: Feraheme® (ferumoxytol) Injectafer® (ferric carboxymaltose) Monoferric® (ferric derisomaltose)* The following -

Methicillin-Resistant Staphylococcus Aureus Infections in County Jails
Prevention, Treatment, and Containment of Methicillin-Resistant Staphylococcus aureus Infections in County Jails Texas Department of State Health Services and Correctional Facilities Workgroup September 2006 2 Clinical guidelines are being made available to the public for informational purposes only. Texas Department of State Health Services (DSHS) does not warrant these guidelines for any other purpose and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. 3 We acknowledge the Federal Bureau of Prisons Clinical Practice Guidelines for the Management Of Methicillin-Resistant Staphylococcus Aureus (MRSA) Infections, August 2005 for general organization and content of this document. 4 Table of Contents Introduction ......................................................................................................................... 6 Colonization ......................................................................................................................... 6 Transmission........................................................................................................................ 7 Screening and Surveillance ................................................................................................ 7 Diagnosis .............................................................................................................................. 8 -

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) ..............................................................................