Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Brunswick Drug Plans Formulary
New Brunswick Drug Plans Formulary August 2019 Administered by Medavie Blue Cross on Behalf of the Government of New Brunswick TABLE OF CONTENTS Page Introduction.............................................................................................................................................I New Brunswick Drug Plans....................................................................................................................II Exclusions............................................................................................................................................IV Legend..................................................................................................................................................V Anatomical Therapeutic Chemical (ATC) Classification of Drugs A Alimentary Tract and Metabolism 1 B Blood and Blood Forming Organs 23 C Cardiovascular System 31 D Dermatologicals 81 G Genito Urinary System and Sex Hormones 89 H Systemic Hormonal Preparations excluding Sex Hormones 100 J Antiinfectives for Systemic Use 107 L Antineoplastic and Immunomodulating Agents 129 M Musculo-Skeletal System 147 N Nervous System 156 P Antiparasitic Products, Insecticides and Repellants 223 R Respiratory System 225 S Sensory Organs 234 V Various 240 Appendices I-A Abbreviations of Dosage forms.....................................................................A - 1 I-B Abbreviations of Routes................................................................................A - 4 I-C Abbreviations of Units...................................................................................A -

How Do We Treat Life-Threatening Anemia in a Jehovah's Witness
HOW DO I...? How do we treat life-threatening anemia in a Jehovah’s Witness patient? Joseph A. Posluszny Jr and Lena M. Napolitano he management of Jehovah’s Witness (JW) The refusal of allogeneic human blood and blood prod- patients with anemia and bleeding presents a ucts by Jehovah’s Witness (JW) patients complicates clinical dilemma as they do not accept alloge- the treatment of life-threatening anemia. For JW neic human blood or blood product transfu- patients, when hemoglobin (Hb) levels decrease Tsions.1,2 With increased understanding of the JW patient beyond traditional transfusion thresholds (<7 g/dL), beliefs and blood product limitations, the medical com- alternative methods to allogeneic blood transfusion can munity can better prepare for optimal treatment of severe be utilized to augment erythropoiesis and restore life-threatening anemia in JW patients. endogenous Hb levels. The use of erythropoietin- Lower hemoglobin (Hb) is associated with increased stimulating agents and intravenous iron has been mortality risk in JW patients. In a study of 300 patients shown to restore red blood cell and Hb levels in JW who refused blood transfusion, for every 1 g/dL decrease patients, although these effects may be significantly in Hb below 8 g/dL, the odds of death increased 2.5-fold delayed. When JW patients have evidence of life- (Fig. 1).3 A more recent single-center update of JW threatening anemia (Hb <5 g/dL), oxygen-carrying patients (n = 293) who declined blood transfusion capacity can be supplemented with the administration reported an overall mortality rate of 8.2%, with a twofold of Hb-based oxygen carriers (HBOCs). -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Venofer ® (Iron Sucrose Injection, USP)
NDA 21-135/S-017 Page 3 Venofer ® (iron sucrose injection, USP) Rx Only DESCRIPTION Venofer® (iron sucrose injection, USP) is a brown, sterile, aqueous, complex of polynuclear iron (III)- hydroxide in sucrose for intravenous use. Iron sucrose injection has a molecular weight of approximately 34,000 – 60,000 daltons and a proposed structural formula: [Na2Fe5O8(OH) ⋅3(H2O)]n ⋅m(C12H22O11) where: n is the degree of iron polymerization and m is the number of sucrose molecules associated with the iron (III)-hydroxide. Each mL contains 20 mg elemental iron as iron sucrose in water for injection. Venofer® is available in 5 mL single dose vials (100 mg elemental iron per 5 mL) and 10 mL single dose vials (200 mg elemental iron per 10 mL). The drug product contains approximately 30% sucrose w/v (300 mg/mL) and has a pH of 10.5- 11.1. The product contains no preservatives. The osmolarity of the injection is 1,250 mOsmol/L. Therapeutic class: Hematinic CLINICAL PHARMACOLOGY Pharmacodynamics: Following intravenous administration of Venofer®, iron sucrose is dissociated by the reticuloendothelial system into iron and sucrose. In 22 hemodialysis patients on erythropoietin (recombinant human erythropoietin) therapy treated with iron sucrose containing 100 mg of iron, three times weekly for three weeks, significant increases in serum iron and serum ferritin and significant decreases in total iron binding capacity occurred four weeks from the initiation of iron sucrose treatment. Pharmacokinetics: In healthy adults treated with intravenous doses of Venofer®, its iron component exhibits first order kinetics with an elimination half-life of 6 h, total clearance of 1.2 L/h, non-steady state apparent volume of distribution of 10.0 L and steady state apparent volume of distribution of 7.9 L. -

Mir-205: a Potential Biomedicine for Cancer Therapy
cells Review miR-205: A Potential Biomedicine for Cancer Therapy Neeraj Chauhan 1,2 , Anupam Dhasmana 1,2, Meena Jaggi 1,2, Subhash C. Chauhan 1,2 and Murali M. Yallapu 1,2,* 1 Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, McAllen, TX 78504, USA; [email protected] (N.C.); [email protected] (A.D.); [email protected] (M.J.); [email protected] (S.C.C.) 2 South Texas Center of Excellence in Cancer Research, School of Medicine, University of Texas Rio Grande Valley, McAllen, TX 78504, USA * Correspondence: [email protected]; Tel.: +1-(956)-296-1734 Received: 3 June 2020; Accepted: 21 August 2020; Published: 25 August 2020 Abstract: microRNAs (miRNAs) are a class of small non-coding RNAs that regulate the expression of their target mRNAs post transcriptionally. miRNAs are known to regulate not just a gene but the whole gene network (signaling pathways). Accumulating evidence(s) suggests that miRNAs can work either as oncogenes or tumor suppressors, but some miRNAs have a dual nature since they can act as both. miRNA 205 (miR-205) is one such highly conserved miRNA that can act as both, oncomiRNA and tumor suppressor. However, most reports confirm its emerging role as a tumor suppressor in many cancers. This review focuses on the downregulated expression of miR-205 and discusses its dysregulation in breast, prostate, skin, liver, gliomas, pancreatic, colorectal and renal cancers. This review also confers its role in tumor initiation, progression, cell proliferation, epithelial to mesenchymal transition, and tumor metastasis. -

Allied Health Professionals Consumer Fact Sheet Board of Registration of Allied Health Professionals
Allied Health Professionals Consumer Fact Sheet Board of Registration of Allied Health Professionals The Board of Registration in Allied Health evaluates the qualifications of applicants for licensure and grants licenses to those who qualify. It establishes rules and regulations to ensure the integrity and competence of licensees. The Board is the link between the consumer and the allied health professional and, as such, promotes the public health, welfare and safety. Allied health professionals are occupational therapists and assistants, athletic trainers, and physical therapists and assistants. Occupational Therapists/Occupational Therapist Assistants Occupational therapists are health professionals who use occupational activities with specific goals in helping people of all ages to prevent, lessen or overcome physical, psychological or developmental disabilities. Occupational Therapists and Occupational Therapist Assistants help people with physical, psychological, or developmental problems regain abilities or adjust to handicaps. They work with physicians, physical and speech therapists, nurses, social workers, psychologists, teachers and other specialists. Patients may face handicaps, injuries, illness, psychological or social problems, or barriers due to age, economic, and cultural factors. Occupational Therapists: Consult with treatment teams to develop individualized treatment programs. Select and teach activities based on the needs and capabilities of each patient Evaluate each patient's progress, attitude and behavior. Design special equipment to aid patients with disabilities. Teach patients how to adjust to home, work, and social environments. Test and evaluate patients' physical and mental abilities. Educate others about occupational therapy. The goal of occupational therapy (OT) is for persons to achieve the highest level of independence after an injury or illness. OT addresses the whole person - cognitive, physical and emotional status. -

Massage Therapy Admission Packet
Caldwell Community College and Technical Institute Welcome to Caldwell Community College and Technical Institute. In this packet, you will find information about our Massage Therapy Program including costs, requirements, and details on how to prepare for your new career. Caldwell Campus 2855 Hickory Blvd Hudson, NC 28638 828.726.2200 Watauga Campus 372 Community College Drive Boone, NC 28607 828.297.8120 Amy Swink Massage Therapy Program Coordinator Watauga Campus E-mail: [email protected] Phone: (828)726-2242 and (828) 297-3811 MASSAGE THERAPY COURSE HEA 3021 This course is designed to prepare the student for the certification examination required for the North Carolina licensure application process. Through class work and practical “hands-on” training, students obtain a solid foundation for professional practice as a Massage Therapist. Upon successful completion of the course, the student is eligible to sit for the state exam and apply for state licensure in North Carolina. Competitive Admissions: Students, who successfully complete Massage Therapy training at CCC&TI, will receive two (2) points toward admission into the Physical Therapy Assistant program at CCC&TI Educational Pathway Example: Massage Therapy training > Licensure by examination > Licensed Massage and Bodywork Therapist Estimated Hourly Earnings: According to the Bureau of Labor Statistics website ( www.bls.gov/oes/current/oes319011.htm), current mean annual wage is $44,950. Employment Opportunities: • Chiropractic offices • Home care agencies • Hospice • Hospitals • Medical offices • Retirement homes • Self-employment • Spas FAQs • Is massage therapy training required to become licensed in North Carolina? o Yes. The North Carolina Board of Massage and Bodywork Therapy (NCBMBT) requires an applicant to successfully complete a state approved training program consisting of a minimum 500 in-class training hours. -

Staphylococcus Aureus Bloodstream Infection Treatment Guideline
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI). The recommendations below are guidelines for care and are not meant to replace clinical judgment. The initial page includes a brief version of the guidance followed by a more detailed discussion of the recommendations with supporting evidence. Also included is an algorithm describing management of patients with blood cultures positive for gram-positive cocci. Brief Key Points: 1. Don’t ignore it – Staphylococcus aureus isolated from a blood culture is never a contaminant. All patients with S. aureus in their blood should be treated with appropriate antibiotics and evaluated for a source of infection. 2. Control the source a. Removing infected catheters and prosthetic devices – Retention of infected central venous catheters and prosthetic devices in the setting of S. aureus bacteremia (SAB) has been associated with prolonged bacteremia, treatment failure and death. These should be removed if medically possible. i. Retention of prosthetic material is associated with an increased likelihood of SAB relapse and removal should be considered even if not clearly infected b. Evaluate for metastatic infections (endocarditis, osteomyelitis, abscesses, etc.) – Metastatic infections and endocarditis are quite common in SAB (11-31% patients with SAB have endocarditis). i. All patients should have a thorough history taken and exam performed with any new complaint evaluated for possible metastatic infection. ii. Echocardiograms should be strongly considered for all patients with SAB iii. All patients with a prosthetic valve, pacemaker/ICD present, or persistent bacteremia (follow up blood cultures positive) should undergo a transesophageal echocardiogram 3. -

Ferric Maltol 30Mg Hard Capsules (Feraccru®) SMC No
ferric maltol 30mg hard capsules (Feraccru®) SMC No. (1202/16) Shield TX UK Limited 04 November 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards and Area Drug and Therapeutic Committees (ADTCs) on its use in Scotland. The advice is summarised as follows: ADVICE: following a full submission ferric maltol (Feraccru®) is not recommended for use within NHS Scotland. Indication under review: in adults for the treatment of iron deficiency anaemia (IDA) in patients with inflammatory bowel disease (IBD). In a pooled analysis of two phase III studies in IBD patients with IDA who had failed previous treatment with oral ferrous products, there was a significantly greater increase in haemoglobin concentrations after 12 weeks of ferric maltol treatment compared with placebo. The submitting company did not present sufficiently robust clinical and economic analyses to gain acceptance by SMC. Overleaf is the detailed advice on this product. Chairman, Scottish Medicines Consortium Published 12 December 2016 1 Indication In adults for the treatment of iron deficiency anaemia (IDA) in patients with inflammatory bowel disease (IBD). Dosing Information One capsule twice daily, morning and evening, on an empty stomach. Treatment duration will depend on severity of iron deficiency but generally at least 12 weeks treatment is required. The treatment should be continued as long as necessary to replenish the body iron stores according to blood tests. Ferric maltol capsules should be taken whole on an empty stomach (with half a glass of water) as the absorption of iron is reduced when it is taken with food. -
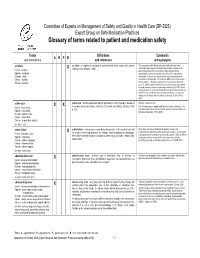
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -
![Medical Synagis (CPT) [RSV-Igim], for Intramuscular Use, 50 (PA) Mg, Each)](https://docslib.b-cdn.net/cover/7463/medical-synagis-cpt-rsv-igim-for-intramuscular-use-50-pa-mg-each-1247463.webp)
Medical Synagis (CPT) [RSV-Igim], for Intramuscular Use, 50 (PA) Mg, Each)
CCHP Code Brand Name Description (PA) = Prior Authorization required Benefit Notes palivizumab (respiratory syncytial virus 90378 immune globulin Medical Synagis (CPT) [RSV-IgIM], for intramuscular use, 50 (PA) mg, each) lutetium lu 177, dotatate, therapeutic, Medical A9513 Lutathera 1 millicureie (PA) Medical A9590 Azedra Iodine I-131, iobenguane, 1 millicurie (PA) radium ra-223 dichloride, therapeutic, Medical A9606 Xofigo per microcurie (PA) in-line cartridge digestive enzyme for B4105 Relizorb Medical enteral feeding each COCAINE HCI NASAL SOL TOP ADMN 1 C9046 Goprelto Medical MG Medical C9047 Cablivi Injection, caplacizumab-yhdp, 1 mg (PA) Injection, romidepsin, non-lyophilized C9065 Medical (e.g. liquid), 1mg injection, belantamab mafodontin- C9069 Blenrep Medical blmf, 0.5 mg C9070 Monjuvi injection, tafasitamab-cxix, 2 mg Medical Medical C9071 Viltepso Injection, viltolarsen, 10 mg (PA) injection, immune globulin (asceniv), Medical C9072 Asceniv 500 mg (PA) Brexucabtagene autoleucel, up to 200 million autologous anti-cd19 car Medical C9073 Tecartus positive viable t cells, including (PA) leukapheresis and dose preparation procedures, per therapeutic dose Effective April 1, 2021 1 CCHP Code Brand Name Description (PA) = Prior Authorization required Benefit Notes prothrombin complex concentrate C9132 Kcentra (human), kcentra, per i.u. of factor ix Medical activity C9248 Cleviprex injection, clevidipine butyrate Medical C9250 Artiss artiss fibrin sealant Medical C9290 Exparel injection, bupivacaine liposome, 1 mg Medical C9293 Voraxaze