Antibiotic Prophylaxis 2017 Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and Its Application
Infect Dis Ther (2017) 6:57–67 DOI 10.1007/s40121-016-0144-8 REVIEW Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and its Application Juwon Yim . Leah M. Molloy . Jason G. Newland Received: November 10, 2016 / Published online: December 30, 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com ABSTRACT infections, CABP caused by penicillin- and ceftriaxone-resistant S. pneumoniae and Ceftaroline is a novel cephalosporin recently resistant Gram-positive infections that fail approved in children for treatment of acute first-line antimicrobial agents. However, bacterial skin and soft tissue infections and limited data are available on tolerability in community-acquired bacterial pneumonia neonates and infants younger than 2 months (CABP) caused by methicillin-resistant of age, and on pharmacokinetic characteristics Staphylococcus aureus, Streptococcus pneumoniae in children with chronic medical conditions and other susceptible bacteria. With a favorable and those with invasive, complicated tolerability profile and efficacy proven in infections. In this review, the microbiological pediatric patients and excellent in vitro profile of ceftaroline, its mechanism of action, activity against resistant Gram-positive and and pharmacokinetic profile will be presented. Gram-negative bacteria, ceftaroline may serve Additionally, clinical evidence for use in as a therapeutic option for polymicrobial pediatric patients and proposed place in therapy is discussed. Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/ 1F47F0601BB3F2DD. Keywords: Antibiotic resistance; Ceftaroline J. Yim (&) fosamil; Children; Methicillin-resistant St. John Hospital and Medical Center, Detroit, MI, Staphylococcus aureus; Streptococcus pneumoniae USA e-mail: [email protected] L. -

FDA-Approved Drugs with Potent in Vitro Antiviral Activity Against Severe Acute Respiratory Syndrome Coronavirus 2
pharmaceuticals Article FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2 1, , 1, 2 1 Ahmed Mostafa * y , Ahmed Kandeil y , Yaseen A. M. M. Elshaier , Omnia Kutkat , Yassmin Moatasim 1, Adel A. Rashad 3 , Mahmoud Shehata 1 , Mokhtar R. Gomaa 1, Noura Mahrous 1, Sara H. Mahmoud 1, Mohamed GabAllah 1, Hisham Abbas 4 , Ahmed El Taweel 1, Ahmed E. Kayed 1, Mina Nabil Kamel 1, Mohamed El Sayes 1, Dina B. Mahmoud 5 , Rabeh El-Shesheny 1 , Ghazi Kayali 6,7,* and Mohamed A. Ali 1,* 1 Center of Scientific Excellence for Influenza Viruses, National Research Centre, Giza 12622, Egypt; [email protected] (A.K.); [email protected] (O.K.); [email protected] (Y.M.); [email protected] (M.S.); [email protected] (M.R.G.); [email protected] (N.M.); [email protected] (S.H.M.); [email protected] (M.G.); [email protected] (A.E.T.); [email protected] (A.E.K.); [email protected] (M.N.K.); [email protected] (M.E.S.); [email protected] (R.E.-S.) 2 Organic & Medicinal Chemistry Department, Faculty of Pharmacy, University of Sadat City, Menoufia 32897, Egypt; [email protected] 3 Department of Biochemistry & Molecular Biology, Drexel University College of Medicine, Philadelphia, PA 19102, USA; [email protected] 4 Department of Microbiology and Immunology, Zagazig University, Zagazig 44519, Egypt; [email protected] 5 Pharmaceutics Department, National Organization for Drug Control and Research, Giza 12654, Egypt; [email protected] 6 Department of Epidemiology, Human Genetics, and Environmental Sciences, University of Texas, Houston, TX 77030, USA 7 Human Link, Baabda 1109, Lebanon * Correspondence: [email protected] (A.M.); [email protected] (G.K.); [email protected] (M.A.A.) Contributed equally to this work. -

National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): a Cross-Sectional Study
Tropical Medicine and Infectious Disease Article National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): A Cross-Sectional Study Joseph Sam Kanu 1,2,* , Mohammed Khogali 3, Katrina Hann 4 , Wenjing Tao 5, Shuwary Barlatt 6,7, James Komeh 6, Joy Johnson 6, Mohamed Sesay 6, Mohamed Alex Vandi 8, Hannock Tweya 9, Collins Timire 10, Onome Thomas Abiri 6,11 , Fawzi Thomas 6, Ahmed Sankoh-Hughes 12, Bailah Molleh 4, Anna Maruta 13 and Anthony D. Harries 10,14 1 National Disease Surveillance Programme, Sierra Leone National Public Health Emergency Operations Centre, Ministry of Health and Sanitation, Cockerill, Wilkinson Road, Freetown, Sierra Leone 2 Department of Community Health, Faculty of Clinical Sciences, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone 3 Special Programme for Research and Training in Tropical Diseases (TDR), World Health Organization, 1211 Geneva, Switzerland; [email protected] 4 Sustainable Health Systems, Freetown, Sierra Leone; [email protected] (K.H.); [email protected] (B.M.) 5 Unit for Antibiotics and Infection Control, Public Health Agency of Sweden, Folkhalsomyndigheten, SE-171 82 Stockholm, Sweden; [email protected] 6 Pharmacy Board of Sierra Leone, Central Medical Stores, New England Ville, Freetown, Sierra Leone; [email protected] (S.B.); [email protected] (J.K.); [email protected] (J.J.); [email protected] (M.S.); [email protected] (O.T.A.); [email protected] (F.T.) Citation: Kanu, J.S.; Khogali, M.; 7 Department of Pharmaceutics and Clinical Pharmacy & Therapeutics, Faculty of Pharmaceutical Sciences, Hann, K.; Tao, W.; Barlatt, S.; Komeh, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown 0000, Sierra Leone 8 J.; Johnson, J.; Sesay, M.; Vandi, M.A.; Directorate of Health Security & Emergencies, Ministry of Health and Sanitation, Sierra Leone National Tweya, H.; et al. -
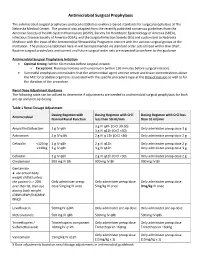
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Sexually Transmitted Diseases Treatment Options
Sexually transmitted disease (STD) treatment options PREFERRED & ALTERNATIVE OPTIONS Many clinical partners are operating in a limited capacity during the COVID-19 pandemic. Below are preferred (in clinic or other location where injections can be given) and alternative (when only oral medicines are available 1) treatments for STDs. Syndrome Preferred Treatments Alternative Treatments Follow-up Male urethritis syndrome Ceftriaxone 250mg intramuscular (IM) x 1 PLUS Men who have sex with men (MSM) and transgender women2: Patients should be counseled to azithromycin 1g PO x 1 Cefixime 800 mg PO x 1 PLUS doxycycline 100 mg PO BID x 7 days be tested for STDs once clinical Presumptively treating: care is resumed in the local If azithromycin is not available: doxycycline 100 Men who have sex with women only: gonorrhea clinics. Clients who have been mg PO BID for 7 days (except in pregnancy3) Cefixime 800mg PO x 1 PLUS azithromycin 1g PO x 1 referred for oral treatment If cephalosporin allergy5 is reported, gentamicin If cefixime is unavailable, substitute cefpodoxime 400mg PO q12h should return for 240mg IM x 1 PLUS azithromycin 2g PO x 1 x 2 for cefixime in above regimens4 comprehensive testing and screening and linked to services If oral cephalosporin not available or history of cephalosporin at that time. allergy5: azithromycin 2g PO x 1 If azithromycin is not available: doxycycline 100 mg PO BID for 7 days (except in pregnancy3) Patients should be advised to abstain from sex for 7 days Treatment typically guided by examination and For presumptive therapy when examination and laboratory following completion of Vaginal discharge syndrome treatment. -

Combination Therapy in Complicated Infections Due to S. Aureus
Combination therapy in complicated infections due to S. aureus Alex Soriano ([email protected]) Service of Infectious Diseases Hospital Clínic of Barcelona ESCMIDUniversity of Barcelona eLibrary IDIBAPS © by author Staphylococcal dissemination to different organs 30 min after i.v. Infection (using a real-time Sureward, et al. imaging) Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary Liver, Kupfer cell (purple ) © byS. aureus author(green) Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 eradication Large cluster of S. from blood aureus inside of KC. Located in phagolysosomes ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary Vancosomes: vancomycin vancomycin (1h before ©or after by) authorwithin liposomes Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 Animal model of S aureus bacteremia. Treatment started after 24h of the infection ESCMID eLibrary single dose © by author ESCMID CaseeLibrary #1 © by author Medical history: A 27 y-o man, without co-morbidity. -

Which Antibiotic Prophylaxis Guidelines for Infective Endocarditis Should Canadian Dentists Follow?
Debate & O P I N I O N Which Antibiotic Prophylaxis Guidelines for Infective Endocarditis Should Canadian Dentists Follow? Contact Author Herve Y. Sroussi, DMD, PhD; Ashwin R. Prabhu, BDS; Dr. Epstein Joel B. Epstein, DMD, MSD, FRCD(C) Email: [email protected] For citation purposes, the electronic version is the definitive version of this article: www.cda-adc.ca/jcda/vol-73/issue-5/401.html ental providers must keep up to date 2 weeks following a procedure, dental proced- with antibiotic prophylaxis guidelines ures conducted even months earlier may be Dfor infectious endocarditis (IE) as these questioned as causative of IE.4 Furthermore, guidelines represent standards of care and periodontal and dental disease increase the determine medicolegal standards. A survey risk of bacteremia with activities of daily of Canadian dentists revealed that practi- living and may more commonly cause bac- tioners tend to employ antibiotic prophylaxis teremia; therefore, good oral care is of para- according to the guidelines in place at the mount importance in patients with conditions time of their graduation from dental school that place them at risk for IE. — guidelines that often do not meet the cur- To reduce the risk of IE following dental rent standards,1 as both the American Heart procedures, prophylactic measures have Association (AHA)2 and the British Society been developed by experts in the fields of for Antimicrobial Chemotherapy (BSAC)3 have microbiology, epidemiology, cardiology and currently updated their recommendations. dentistry. The principle preventive measure Host factors that increase the risk of IE recommended is the use of prophylactic anti- include specific cardiac abnormalities, a biotics before certain dental procedures in pa- previous episode of IE and the extent of the tients identified as at risk. -

Azithromycin (Systemic) | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Azithromycin (Systemic) This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Zithromax; Zithromax Tri-Pak; Zithromax Z-Pak; Zmax [DSC] Brand Names: Canada ACT Azithromycin [DSC]; AG-Azithromycin; APO-Azithromycin; APO-Azithromycin Z; AURO-Azithromycin; DOM-Azithromycin; GD-Azithromycin [DSC]; GEN- Azithromycin; JAMP-Azithromycin; M-Azithromycin; Mar-Azithromycin; MYLAN- Azithromycin [DSC]; NRA-Azithromycin; PHL-Azithromycin [DSC]; PMS- Azithromycin; PRO-Azithromycin; RATIO-Azithromycin; RIVA-Azithromycin; SANDOZ Azithromycin; TEVA-Azithromycin; Zithromax What is this drug used for? It is used to treat or prevent bacterial infections. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have turned yellow or had liver side effects with this drug before. If you have any of these health problems: Long QTc on ECG or other heartbeat that is not normal, slow heartbeat, or low potassium or magnesium levels. If you have heart failure (weak heart). Azithromycin (Systemic) 1/8 If you have ever had a certain type of abnormal heartbeat (torsades de pointes). If you are taking any drugs that can cause a certain type of heartbeat that is not normal (prolonged QT interval). There are many drugs that can do this. Ask your doctor or pharmacist if you are not sure. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

Identification of Macrolide Antibiotic-Binding Human P8 Protein
J. Antibiot. 61(5): 291–296, 2008 THE JOURNAL OF ORIGINAL ARTICLE ANTIBIOTICS Identification of Macrolide Antibiotic-binding Human_p8 Protein Tetsuro Morimura, Mio Hashiba, Hiroshi Kameda, Mihoko Takami, Hirokazu Takahama, Masahiko Ohshige, Fumio Sugawara Received: December 10, 2007 / Accepted: May 1, 2008 © Japan Antibiotics Research Association Abstract Clarithromycin is a macrolide antibiotic that is widely used in clinical medicine. Macrolide antibiotics Introduction such as clarithromycin specifically bind to the 50S subunit of the bacterial ribosome thereby interfering with protein Launched in 1990 by Abbott Laboratories, clarithromycin biosynthesis. A selected peptide sequence from our former A (CLA: synonym of 6-O-methylerythromycin, 1) [1] is a study, composed of 19 amino acids, which was isolated macrolide antibiotic that is commonly used to treat a from a phage display library because of its ability to bind variety of human bacterial infections. Macrolide clarithromycin, displayed significant similarity to a portion antibiotics, represented by erythromycin A (ERY, 2) and its of the human_p8 protein. The recombinant p8 protein structural homologues, specifically bind to the bacterial binds to biotinylated-clarithromycin immobilized on a 50S ribosomal subunit resulting in the inhibition of streptavidin-coated sensor chip and the dissociation bacterial protein biosynthesis [2]. CLA (1) is also constant was determined. The binding of recombinant p8 commonly used to target gastric Helicobacter pylori, which protein to double-stranded DNA was inhibited by is implicated in both gastric ulcers and cancer [3]. biotinylated-clarithromycin, clarithromycin, erythromycin Numerous studies to develop novel pharmaceutical and azithromycin in gel mobility shift assay. applications for macrolide antibiotics, including CLA (1), Dechlorogriseofulvin, obtained from a natural product ERY (2) and azithromycin (AZM, 3), have been published screening, also inhibited human p8 protein binding to [4]. -

Development of a Firefly Luciferase-Based Assay For
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Veterinary and Biomedical Sciences, Papers in Veterinary and Biomedical Science Department of February 1999 Development of a Firefly ucifL erase-Based Assay for Determining Antimicrobial Susceptibility of Mycobacterium avium subsp. paratuberculosis Stephanie L. Williams University of Nebraska - Lincoln N. Beth Harris University of Nebraska - Lincoln Raul G. Barletta University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/vetscipapers Part of the Veterinary Medicine Commons Williams, Stephanie L.; Harris, N. Beth; and Barletta, Raul G., "Development of a Firefly ucifL erase-Based Assay for Determining Antimicrobial Susceptibility of Mycobacterium avium subsp. paratuberculosis" (1999). Papers in Veterinary and Biomedical Science. 8. https://digitalcommons.unl.edu/vetscipapers/8 This Article is brought to you for free and open access by the Veterinary and Biomedical Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Veterinary and Biomedical Science by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 1999, p. 304–309 Vol. 37, No. 2 0095-1137/99/$04.0010 Copyright © 1999, American Society for Microbiology. All Rights Reserved. Development of a Firefly Luciferase-Based Assay for Determining Antimicrobial Susceptibility of Mycobacterium avium subsp. paratuberculosis 1 1 1,2 STEPHANIE L. WILLIAMS, N. BETH HARRIS, * AND RAU´ L G. BARLETTA * Department of Veterinary and Biomedical Sciences1 and Center for Biotechnology,2 University of Nebraska, Lincoln, Nebraska 68583-0905 Received 29 June 1998/Returned for modification 21 October 1998/Accepted 21 October 1998 Paratuberculosis (Johne’s disease) is a fatal disease of ruminants for which no effective treatment is avail- able. -

A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients
DOI: 10.14744/ejmo.2021.16263 EJMO 2021;5(1):63–70 Research Article A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients Abu Taiub Mohammed Mohiuddin Chowdhury,1 Mohammad Shahbaz,2 Md Rezaul Karim,3 Jahirul Islam, Guo Dan,1 Shuixiang He1 1Department of Gastroenterology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, P.R. China 2Chakoria Upazilla Health Complex, Cox’s Bazar, Bangladesh 3Biomedical Research Institute of Hubei University of Medicine, Shiyan, China 4Department of Epidemiology and Health Statistics, Xi’an Jiaotong University, Xi’an, Shaanxi, P.R. China Abstract Objectives: We investigated the outcomes of Ivermectin-Doxycycline vs. Hydroxychloroquine-Azithromycin combina- tion therapy in mild to moderate COVID19 patients. Methods: Patients were divided randomly into two groups: Ivermectin 200µgm/kg single dose + Doxycycline 100mg BID for ten days in group A, and Hydroxychloroquine 400mg for the first day, then 200mg BID for nine days + Azithro- mycin 500mg daily for five days in group B (Control group). RT-PCR for SARS-CoV-2 infection was repeated in all symp- tomatic patients on the second day onward without symptoms. Repeat PCR was done every two days onward if the result found positive. Time to the negative PCR and symptomatic recovery was measured for each group. Results: All subjects in Group A reached a negative PCR, at a mean of 8.93 days, and reached symptomatic recovery, at a mean of 5.93 days, with 55.10% symptom-free by the fifth day. In group B, 96.36% reached a negative PCR at a mean of 9.33 days and were symptoms-free at 6.99 days.