Ranvet's Filybol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Rules Pertaining to the Banning of Anabolic Steroids in the Western Australian Harness Racing Industry to Be Introduced 1St September 2014
NEW RULES PERTAINING TO THE BANNING OF ANABOLIC STEROIDS IN THE WESTERN AUSTRALIAN HARNESS RACING INDUSTRY TO BE INTRODUCED 1ST SEPTEMBER 2014 Notice is hereby given that the Board of Racing and Wagering WA have resolved that the RWWA Rules of Harness Racing 2004 be amended. In accordance with section 45 (1) (b) of the Racing and Wagering Western Australia Act 2003 the Board of Racing and Wagering WA on the 10th April 2014 resolved that these amendments be adopted accordingly into the RWWA Rules of Harness Racing. The Harness Racing Board had advised of these amendments and the RWWA Board has determined that these amendments will come into effect on 1st September 2014. The details of the relevant rules pertaining to this ban of anabolic steroids for reference can be found following this advice. There are many implications arising from the introduction of these rules, and to assist trainers and veterinarians to comply with the new rules the following explanatory statement has been prepared. Which steroids are banned under these rules? The new rules ban the use of "anabolic androgenic steroids" in Standardbred horses at any time from birth until retirement. "Anabolic androgenic steroids" include those that are currently registered in Australia by the APVMA for use in horses, such as boldenone, ethylestrenol (in Nitrotain), methandriol, nandrolone, stanozolol and testosterone. Exogenous anabolic androgenic steroids that are banned also include but are not limited to those listed in the WADA prohibited list, such as 1-androstenediol; 1-androstenedione; -

Part I Biopharmaceuticals
1 Part I Biopharmaceuticals Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 3 1 Analogs and Antagonists of Male Sex Hormones Robert W. Brueggemeier The Ohio State University, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Columbus, Ohio 43210, USA 1Introduction6 2 Historical 6 3 Endogenous Male Sex Hormones 7 3.1 Occurrence and Physiological Roles 7 3.2 Biosynthesis 8 3.3 Absorption and Distribution 12 3.4 Metabolism 13 3.4.1 Reductive Metabolism 14 3.4.2 Oxidative Metabolism 17 3.5 Mechanism of Action 19 4 Synthetic Androgens 24 4.1 Current Drugs on the Market 24 4.2 Therapeutic Uses and Bioassays 25 4.3 Structure–Activity Relationships for Steroidal Androgens 26 4.3.1 Early Modifications 26 4.3.2 Methylated Derivatives 26 4.3.3 Ester Derivatives 27 4.3.4 Halo Derivatives 27 4.3.5 Other Androgen Derivatives 28 4.3.6 Summary of Structure–Activity Relationships of Steroidal Androgens 28 4.4 Nonsteroidal Androgens, Selective Androgen Receptor Modulators (SARMs) 30 4.5 Absorption, Distribution, and Metabolism 31 4.6 Toxicities 32 Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 4 Analogs and Antagonists of Male Sex Hormones 5 Anabolic Agents 32 5.1 Current Drugs on the Market 32 5.2 Therapeutic Uses and Bioassays -

Ranvet's Filybol
Ranvet's Filybol Ranvet Chemwatch Hazard Alert Code: 2 Chemwatch: 4787-83 Issue Date: 08/02/2016 Version No: 5.1.1.1 Print Date: 01/24/2018 Safety Data Sheet according to WHS and ADG requirements S.GHS.AUS.EN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING Product Identifier Product name Ranvet's Filybol Chemical Name peanut oil Synonyms Not Available Other means of Not Available identification Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Non-virilising anabolic combination for fillies, mares, colts and stallions. Details of the supplier of the safety data sheet Registered company Ranvet name Address 10-12 Green Street Banksmeadow NSW 2019 Australia Telephone +61 2 9666 1744 Fax +61 2 9666 1755 Website https://www.ranvet.com.au/other_msds.htm Email [email protected] Emergency telephone number Association / Not Available Organisation Emergency telephone +61 425 061 584 numbers Other emergency Not Available telephone numbers SECTION 2 HAZARDS IDENTIFICATION Classification of the substance or mixture Poisons Schedule S4 Carcinogenicity Category 2, Reproductive Toxicity Category 2, Acute Aquatic Hazard Category 2, Chronic Aquatic Hazard Classification [1] Category 2 1. Classified by Chemwatch; 2. Classification drawn from HSIS ; 3. Classification drawn from EC Directive 1272/2008 - Legend: Annex VI Label elements Hazard pictogram(s) SIGNAL WORD WARNING Continued... Chemwatch: 4787-83 Page 2 of 10 Issue Date: 08/02/2016 Version No: 5.1.1.1 Ranvet's Filybol Print Date: 01/24/2018 Hazard statement(s) H351 Suspected of causing cancer. H361 Suspected of damaging fertility or the unborn child. -
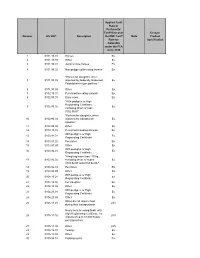
Number HS 2007 Description Applied Tariff Rate Or Preferential Tariff Rate
Applied Tariff Rate or Preferential Tariff Rate over Ex-out / Number HS 2007 Description the NMF Tariff Note Product Rate for Specification Colombia under the FTA since 2010 1 0101.10.01 Horses Ex. 2 0101.10.99 Other Ex. 3 0101.90.01 Jump or race horses Ex. 4 0101.90.02 Non pedigreed breeding horses Ex. "Horses for slaughter, when 5 0101.90.03 imported by Federally Inspected Ex. Establishment type packers." 6 0101.90.99 Other Ex. 7 0102.10.01 Pure-bred breeding animals Ex. 8 0102.90.01 Dairy cows Ex. "With pedigree or High Registrating Certificate, 9 0102.90.02 Ex. excluding those of code 0102.90.01" "Bovines for slaughter, when 10 0102.90.03 imported by Industrial de Ex. Abastos." 11 0102.90.99 Other Ex. 12 0103.10.01 Pure-bred breeding animals Ex. With pedigree or High 13 0103.91.01 Ex. Registrating Certificate. 14 0103.91.02 Peccaries Ex. 15 0103.91.99 Other Ex. With pedigree or High 16 0103.92.01 Ex. Registrating Certificate. "Weighing more than 110 kg., 17 0103.92.02 excluding those of codes Ex. 0103.92.01 and 0103.92.03." 18 0103.92.03 Peccaries Ex. 19 0103.92.99 Other Ex. With pedigree or High 20 0104.10.01 Ex. Registrating Certificate. 21 0104.10.02 For slaughter. Ex. 22 0104.10.99 Other Ex. With pedigree or High 23 0104.20.01 Ex. Registrating Certificate. 24 0104.20.99 Other Ex. When do not require food 25 0105.11.01 28% during their transportation Newly born breeding birds, with High Registrating Certificate, for 26 0105.11.02 28% imports of up to 18,000 heads per transaction. -

Permanent Ban on Anabolic Androgenic Steroids From
Permanent Ban on Anabolic Androgenic Steroids to be introduced into the Greyhounds Australasia Rules Warning to trainers – Anabolic Androgenic Steroids (AAS) usage in greyhounds On 1 January 2016, Greyhounds Australasia will introduce Anabolic Androgenic Steroids to the list of Permanently Banned Prohibited Substances within GAR 79A (2) xx. as follows: “Anabolic androgenic steroids excluding those that are defined as an exempted substance pursuant to GAR 1.” The relevant exempted substance listed within GAR 1 is as follows: “Ethyloestrenol when administered orally to a greyhound bitch and where it has been prescribed by a veterinary surgeon for the sole purpose of regulating or preventing oestrus in that bitch.” Participants are advised that in accordance with GAR 79A they must never possess, acquire, attempt to acquire, administer or allow to be administered to any greyhound from birth until retirement, any anabolic androgenic steroid, excluding ethyloestrenol where it is appropriately prescribed for use as an oestrous suppressant in females. “Anabolic androgenic steroids” include those that are currently registered in Australia by the APVMA such as boldenone, ethyloestrenol, methandriol, nandrolone, stanozolol and testosterone. Others include but are not limited to 1- androstenediol; 1-androstenedione; bolandiol; bolasterone; boldione; calusterone; clostebol; danazol; dehydrochlormethyltestosterone; desoxymethyltestosterone; drostanolone; fluoxymesterone; formebolone; furazabol; gestrinone; 4-hydroxytestosterone; mestanolone; mesterolone; -

A28 Anabolic Steroids
Anabolic SteroidsSteroids A guide for users & professionals his booklet is designed to provide information about the use of anabolic steroids and some of the other drugs Tthat are used in conjunction with them. We have tried to keep the booklet free from technical jargon but on occasions it has proven necessary to include some medical, chemical or biological terminology. I hope that this will not prevent the information being accessible to all readers. The first section explaining how steroids work is the most complex, but it gets easier to understand after that (promise). The booklet is not intended to encourage anyone to use these drugs but provides basic information about how they work, how they are used and the possible consequences of using them. Anabolic Steroids A guide for users & professionals Contents Introduction .........................................................7 Formation of Testosterone ...............................................................8 Method of Action ..............................................................................9 How Steroids Work (illustration).......................................................10 Section 1 How Steroids are Used ....................................... 13 What Steroid? ................................................................................ 14 How Much to Use? ......................................................................... 15 Length of Courses? ........................................................................ 15 How Often to Use Steroids? -

LEGISLATIVE ASSEMBLY Question on Notice
LEGISLATIVE ASSEMBLY Question On Notice Thursday, 15 February 2018 2560. Ms M. M Quirk to the Minister for Police; I refer to the commencement of operation of the Road Traffic Amendment Act 2016 in March 2017 which introduced compulsory blood or urine samples to be taken from drivers involved in serious crashes, and I ask: (a) since March 2017 how many such samples have been taken; (b) for what specific substances are those blood or urine samples tested; (c) what are the results of those tests to date; and (d) what percentage of drivers have been found to have ingested more than one substance capable of impairing driving skills? Answer (a) The compulsory taking of blood from all drivers involved in serious crashes commenced on 10 March 2017. Between 10 March 2017 and 22 February 2018 (inclusive) a total of 398 blood test samples have been collected under the provision of the Road Traffic Amendment Act 2016. There have been no urine tests collected. (b) Please see attached table for a list of substances in blood sample that are identifiable in ChemCentre toxicology analysis (Paper Number). (c) Of the 398 blood samples collected, 48 are pending results of ChemCentre analysis. Of the 350 analysed, 259 samples had a specific substance(s) detected and 91 samples had no specific substance detected. d) Of the 259 samples with specific substance(s) detected, 92% were found to have multiple substances (more than one). Detectable Substances in Blood Samples capable of identification by the ChemCentre WA. ACETALDEHYDE AMITRIPTYLINE/NORTRIPTYLINE -

2004 Prohibited List
The World Anti-Doping Code THE 2004 PROHIBITED LIST INTERNATIONAL STANDARD (update 17 March 2004) This List shall come into effect on 26 March 2004. THE 2004 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 26 March 2004 (Updated 17 March 2004) SUBSTANCES AND METHODS PROHIBITED IN-COMPETITION PROHIBITED SUBSTANCES S1. STIMULANTS The following stimulants are prohibited, including both their optical (D- and L-) isomers where relevant: Adrafinil, amfepramone, amiphenazole, amphetamine, amphetaminil, benzphetamine, bromantan, carphedon, cathine*, clobenzorex, cocaine, dimethylamphetamine, ephedrine**, etilamphetamine, etilefrine, fencamfamin, fenetylline, fenfluramine, fenproporex, furfenorex, mefenorex, mephentermine, mesocarb, methamphetamine, methylamphetamine, methylenedioxyamphetamine, methylenedioxymethamphetamine, methylephedrine**, methylphenidate, modafinil, nikethamide, norfenfluramine, parahydroxyamphetamine, pemoline, phendimetrazine, phenmetrazine, phentermine, prolintane, selegiline, strychnine, and other substances with similar chemical structure or similar pharmacological effect(s)***. * Cathine is prohibited when its concentration in urine is greater than 5 micrograms per millilitre. ** Each of ephedrine and methylephedrine is prohibited when its concentration in urine is greater than 10 micrograms per millilitre. *** The substances included in the 2004 Monitoring Program are not considered as Prohibited Substances. The Prohibited List 2004 1 26 March 2004 S2. NARCOTICS The following narcotics are prohibited: buprenorphine, dextromoramide, -

Georgia State Forensic Drugs
Comprehensive Forensic FT-IR Collection Library Listing – 4,286 spectra This extensive library contains materials not only of forensic interest but also for general problem solving and identification of unknown substances in industry and academia. The wide range of items include drugs, clandestine lab chemicals, explosives, paints, fabrics, dyes, polymers, inorganic compounds, pigments, adhesives, and other common materials. The library consists of 4,286 spectra that were acquired from a wide range of laboratories involved in forensic investigations. The collection includes the following classes of compounds: • Drugs of abuse, scheduled materials • Pharmaceuticals, vitamins and excipients • Clandestine lab materials and intermediates • Solvents, organic chemicals and hazardous chemicals • Accelerants • Lubricants and natural oils • Explosives, pyrotechnics, primers, powders and boosters • Herbal and plant material and fibers • Automobile paint vehicles, pigments, primers and clear coats • Textiles, natural and man-made fibers, carpet materials • Paints, coatings, varnishes, oils • Dyes and stains • Polymers, monomers, copolymers, plasticizers and rubbers • Inorganics, pigments, minerals and clays • Tape, adhesives, sealants, glues, caulks and putties • Crystal test derivatives and intermediates • Household chemicals, cleaning agents, surfactants and pesticide All spectra were measured using micro or macro Diamond ATR, thin films on salt windows or KBr pellets at 4 cm-1 spectral resolution. Comprehensive Forensic FT-IR Collection Index -

Gas Chromatography for the Analysis of Drugs Michelle L
Seton Hall University eRepository @ Seton Hall Seton Hall University Dissertations and Theses Seton Hall University Dissertations and Theses (ETDs) Spring 5-16-2015 QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) Extraction – Gas Chromatography for the Analysis of Drugs Michelle L. Schmidt [email protected] Follow this and additional works at: https://scholarship.shu.edu/dissertations Part of the Analytical Chemistry Commons, and the Medicinal-Pharmaceutical Chemistry Commons Recommended Citation Schmidt, Michelle L., "QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) Extraction – Gas Chromatography for the Analysis of Drugs" (2015). Seton Hall University Dissertations and Theses (ETDs). 2060. https://scholarship.shu.edu/dissertations/2060 QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) Extraction – Gas Chromatography for the Analysis of Drugs BY Michelle L. Schmidt DISSERTATION Submitted to the Department of Chemistry and Biochemistry at Seton Hall University in partial fulfillment of the requirements for the degree of Doctor of Philosophy. May, 2015 1 © Michelle L. Schmidt 2015 2 We certify that we have read this dissertation and that in our opinion it is adequate to scientific scope and quality as a dissertation for the degree of Doctor of Philosophy. APPROVED _________________________________________________________ Nicholas H. Snow, Ph.D Research Mentor and Chair, Department of Chemistry and Biochemistry _________________________________________________________ Yuri V. Kazakevich, Ph.D Member of the Dissertation Committee _________________________________________________________ Sergiu M. Gorun, Ph.D Member of the Dissertation Committee 3 ACKNOWLEDGEMENTS I would like to thank Dr. Nicholas H. Snow for his continued guidance, input, and advice throughout my time at Seton Hall University and whose help was invaluable and deeply appreciated. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Pharmacy Data Management Drug Exception List
Pharmacy Data Management Drug Exception List Patch PSS*1*127 updated the following drugs with the listed NCPDP Multiplier and NCPDP Dispense Unit. These two fields were added as part of this patch to the DRUG file (#50). Please refer to the Release notes for ePharmacy/ECME Enhancements for Pharmacy Release Notes (BPS_1_5_EPHARMACY_RN_0907.PDF) on the VistA Documentation Library (VDL). The IEN column reflects the IEN for the VA PRODUCT file (#50.68). The ePharmacy Change Control Board provided the following list of drugs with the specified NCPDP Multiplier and NCPDP Dispense Unit values. This listing was used to update the DRUG file (#50) with a post install routine in the PSS*1*127 patch. NCPDP File 50.68 NCPDP Dispense IEN Product Name Multiplier Unit 2 ATROPINE SO4 0.4MG/ML INJ 1.00 ML 3 ATROPINE SO4 1% OINT,OPH 3.50 GM 6 ATROPINE SO4 1% SOLN,OPH 1.00 ML 7 ATROPINE SO4 0.5% OINT,OPH 3.50 GM 8 ATROPINE SO4 0.5% SOLN,OPH 1.00 ML 9 ATROPINE SO4 3% SOLN,OPH 1.00 ML 10 ATROPINE SO4 2% SOLN,OPH 1.00 ML 11 ATROPINE SO4 0.1MG/ML INJ 1.00 ML 12 ATROPINE SO4 0.05MG/ML INJ 1.00 ML 13 ATROPINE SO4 0.4MG/0.5ML INJ 1.00 ML 14 ATROPINE SO4 0.5MG/ML INJ 1.00 ML 15 ATROPINE SO4 1MG/ML INJ 1.00 ML 16 ATROPINE SO4 2MG/ML INJ 1.00 ML 18 ATROPINE SO4 2MG/0.7ML INJ 0.70 ML 21 ATROPINE SO4 0.3MG/ML INJ 1.00 ML 22 ATROPINE SO4 0.8MG/ML INJ 1.00 ML 23 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,5ML 5.00 ML 24 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,10ML 10.00 ML 25 ATROPINE SO4 1MG/ML INJ,AMP,1ML 1.00 ML 26 ATROPINE SO4 0.2MG/0.5ML INJ,AMP,0.5ML 0.50 ML 30 CODEINE PO4 30MG/ML