9458 Federal Register / Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -
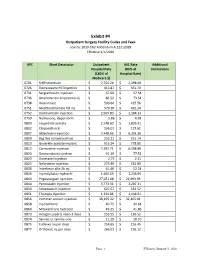
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

Oregon Health Authority Division of Medical Assistance Programs Addendum a - Final OPPS Apcs for CY 2012 Effective October 1, 2012
Oregon Health Authority Division of Medical Assistance Programs Addendum A - Final OPPS APCs for CY 2012 Effective October 1, 2012 Relative APC Group Title SI Weight 0001 Level I Photochemotherapy S 0.5042 0002 Fine Needle Biopsy/Aspiration T 1.6115 0003 Bone Marrow Biopsy/Aspiration T 3.5702 0004 Level I Needle Biopsy/ Aspiration Except Bone Marrow T 4.5746 0005 Level II Needle Biopsy/Aspiration Except Bone Marrow T 8.1566 0006 Level I Incision & Drainage T 1.4206 0007 Level II Incision & Drainage T 13.1250 0008 Level III Incision and Drainage T 20.5648 0012 Level I Debridement & Destruction T 0.3878 0013 Level II Debridement & Destruction T 0.8785 0015 Level III Debridement & Destruction T 1.4989 0016 Level IV Debridement & Destruction T 2.7592 0017 Level V Debridement & Destruction T 21.6661 0019 Level I Excision/ Biopsy T 4.4238 0020 Level II Excision/ Biopsy T 8.2746 0021 Level III Excision/ Biopsy T 17.0074 0022 Level IV Excision/ Biopsy T 23.2662 0028 Level I Breast Surgery T 25.5054 0029 Level II Breast Surgery T 33.4070 0030 Level III Breast Surgery T 44.8999 0031 Smoking Cessation Services X 0.2997 0034 Mental Health Services Composite S 2.7295 0035 Vascular Puncture and Minor Diagnostic Procedures X 0.2691 0037 Level IV Needle Biopsy/Aspiration Except Bone Marrow T 15.3499 0039 Level I Implantation of Neurostimulator Generator S 216.7598 0040 Level I Implantation/Revision/Replacement of Neurostimulator Electrodes S 63.7616 0041 Level I Arthroscopy T 29.6568 0042 Level II Arthroscopy T 57.0137 0045 Bone/Joint Manipulation Under -

Original New Drug Applications
New Drugs Reviewed by CPG January 23, 2017 (Original New Drug Applications: FDA) Generic Name Trade Name Indication(s) CPG Action Immune globulin Cuvitru Biologic and In accordance with subcutaneous Immunological the SCA [human] 20% solution Agents/Immune Globulins/ Immune Globulin (Human) subcutaneous. Indicated as replacement for primary humoral immunodeficiency in adult and pediatric patients age two and older. eteplirsen Exondys 51 Central Nervous System In accordance with Agents/Antisense the SCA Oligonucleotides. Treatment of patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. First drug approved for reatment of patients with Duchenne muscular dystrophy. levonorgestrel- Kyleena Endocrine and May prescribe releasing intrauterine Metabolic Agents/sex system 19.5 mg homones/contraceptive Hormones. Prevention of pregnancy for up to five years. **Phentermine Lomaira Central Nervous System In accordance with hydrochloride 8 mg Agents/ the SCA anorexiants/sympathomi metic anorexiants. Short term use weight reduction in adults with **(new formulation at an initial BMI of 30 or lower dosage) more or 27 with at least one weight-related condition. 1 New Drugs January 2017 canaglifozin/ Invokamet XR Endocrine and May prescribe metformin HCL Metabolic Agents/ extended-release antidiabetic agents/antidiabetic combination products. Treatment of adults with type 2 diabetes as an adjunct to diet and exercise. Adalimumab-atto Amjevita Biologic and In accordance with Immunological Agents/ the SCA Immunologic Agents/Immunomodulat ors/Tumor necrosis Factor-Alpha Blockers. Indicated for treatment of adults with: rheumatoid arthritis, psoriatic arthritis,ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis. Also indicated for juvenile idiopathic arthritis in pts age 4 years and older. -

Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients with Breast Cancer: Estimates from SEER-Medicare Data
Supplemental online content for: Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients With Breast Cancer: Estimates From SEER-Medicare Data Anne A. Eaton, MS; Camelia S. Sima, MD, MS; and Katherine S. Panageas, DrPH J Natl Compr Canc Netw 2016;14(1):57–65 • eAppendix 1: J-Codes Representing Intravenous Chemotherapy • eAppendix 2: Established Sequential Adjuvant Chemotherapy Regimens for Breast Cancer • eTable 1: Patient Characteristics © JNCCN—Journal of the National Comprehensive Cancer Network | Volume 14 Number 1 | January 2016 Eaton et al - 1 eAppendix 1: J-Codes Representing Intravenous Chemotherapy J-Code Agent J-Code Agent J9000 Injection, doxorubicin HCl, 10 mg J9165 Injection, diethylstilbestrol diphosphate, 250 J9001 Injection, doxorubicin HCl, all lipid mg formulations, 10 mg J9170 Injection, docetaxel, 20 mg J9010 Injection, alemtuzumab, 10 mg J9171 Injection, docetaxel, 1 mg J9015 Injection, aldesleukin, per single use vial J9175 Injection, Elliotts’ B solution, 1 ml J9017 Injection, arsenic trioxide, 1 mg J9178 Injection, epirubicin HCl, 2 mg J9020 Injection, asparaginase, 10,000 units J9179 Injection, eribulin mesylate, 0.1 mg J9025 Injection, azacitidine, 1 mg J9180 Epirubicin HCl, 50 mg J9027 Injection, clofarabine, 1 mg J9181 Injection, etoposide, 10 mg J9031 BCG (intravesical) per instillation J9182 Etoposide, 100 mg J9033 Injection, bendamustine HCl, 1 mg J9185 Injection, fludarabine phosphate, 50 mg J9035 Injection, bevacizumab, 10 mg J9190 Injection, fluorouracil, 500 mg J9040 Injection, -

Standard Oncology Criteria C16154-A
Prior Authorization Criteria Standard Oncology Criteria Policy Number: C16154-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE DUE BEFORE 03/2016 12/2/2020 1/26/2022 HCPCS CODING TYPE OF CRITERIA LAST P&T APPROVAL/VERSION N/A RxPA Q1 2021 20210127C16154-A PRODUCTS AFFECTED: See dosage forms DRUG CLASS: Antineoplastic ROUTE OF ADMINISTRATION: Variable per drug PLACE OF SERVICE: Retail Pharmacy, Specialty Pharmacy, Buy and Bill- please refer to specialty pharmacy list by drug AVAILABLE DOSAGE FORMS: Abraxane (paclitaxel protein-bound) Cabometyx (cabozantinib) Erwinaze (asparaginase) Actimmune (interferon gamma-1b) Calquence (acalbrutinib) Erwinia (chrysantemi) Adriamycin (doxorubicin) Campath (alemtuzumab) Ethyol (amifostine) Adrucil (fluorouracil) Camptosar (irinotecan) Etopophos (etoposide phosphate) Afinitor (everolimus) Caprelsa (vandetanib) Evomela (melphalan) Alecensa (alectinib) Casodex (bicalutamide) Fareston (toremifene) Alimta (pemetrexed disodium) Cerubidine (danorubicin) Farydak (panbinostat) Aliqopa (copanlisib) Clolar (clofarabine) Faslodex (fulvestrant) Alkeran (melphalan) Cometriq (cabozantinib) Femara (letrozole) Alunbrig (brigatinib) Copiktra (duvelisib) Firmagon (degarelix) Arimidex (anastrozole) Cosmegen (dactinomycin) Floxuridine Aromasin (exemestane) Cotellic (cobimetinib) Fludara (fludarbine) Arranon (nelarabine) Cyramza (ramucirumab) Folotyn (pralatrexate) Arzerra (ofatumumab) Cytosar-U (cytarabine) Fusilev (levoleucovorin) Asparlas (calaspargase pegol-mknl Cytoxan (cyclophosphamide) -

PHOTOFRIN® Sterile Porfimer Sodium for Injection For
PHOTOFRIN® Sterile Porfimer Sodium for Injection For Intravenous Use Antineoplastic Photosensitizing Agent Concordia Laboratories Inc. DATE OF PREPARATION: 5 Canewood Industrial Park October 27, 2014 St. Michael, St. Michael, Barbados BB11005 CONTROL # 178591 PHOTOFRIN® Product Monograph PRODUCT MONOGRAPH PHOTOFRIN® Sterile Porfimer Sodium for Injection For Intravenous Use ANTINEOPLASTIC PHOTOSENSITIZING AGENT CAUTION: PHOTOFRIN® IS AN INJECTABLE PHOTOSENSITIZING DRUG FOR USE IN PHOTODYNAMIC THERAPY (PDT) FOR TREATMENT OF CANCER AND PRE-MALIGNANT CONDITIONS (E.G., HIGH-GRADE DYSPLASIA IN BARRETT'S ESOPHAGUS). PHOTODYNAMIC THERAPY IS A PHOTOCHEMICAL PROCESS REQUIRING SPECIFIC LASERS AND FIBER OPTICS. PHOTODYNAMIC THERAPY WITH PHOTOFRIN® SHOULD ONLY BE APPLIED BY PHYSICIANS TRAINED IN THE ENDOSCOPIC USE OF PDT WITH PHOTOFRIN® AND ONLY IN THOSE FACILITIES PROPERLY EQUIPPED FOR THE PROCEDURE. PHOTOFRIN® MAY CAUSE RESIDUAL PHOTOSENSITIVITY FOR 30 DAYS OR MORE RESULTING IN ERYTHEMA AND BLISTERING OF THE SKIN WHEN IT IS EXPOSED TO DIRECT SUNLIGHT OR BRIGHTLY FOCUSED INDOOR LIGHT (E.G., FROM EXAMINATION 2 PHOTOFRIN® Product Monograph LAMPS, OPERATING ROOM LAMPS, FLOODLIGHTS, HALOGEN LAMPS, ETC.). ACTION & CLINICAL PHARMACOLOGY Pharmacodynamics The cytotoxic and antitumor actions of PHOTOFRIN® (porfimer sodium) are light and oxygen dependent. Photodynamic therapy (PDT) with PHOTOFRIN® is a 2-stage process. The first stage is the intravenous injection of PHOTOFRIN®. Clearance from a variety of tissues occurs over 40-72 hours; but tumor, skin, and organs of the reticuloendothelial system (including liver and spleen) retain PHOTOFRIN® for a longer period. Illumination with 630 nm wavelength laser light constitutes the second and final stage of therapy. Tumor selectivity in treatment occurs through a combination of selective retention of PHOTOFRIN® and selective delivery of light. -

Treating Non-Small Cell Lung Cancer
cancer.org | 1.800.227.2345 Treating Non-Small Cell Lung Cancer If you've been diagnosed with non-small cell lung cancer (NSCLC), your cancer care team will discuss your treatment options with you. It's important to weigh the benefits of each treatment option against the possible risks and side effects. How is non-small cell lung cancer treated? Treatments for NSCLC can include: ● Surgery for Non-Small Cell Lung Cancer ● Radiofrequency Ablation (RFA) for Non-Small Cell Lung Cancer ● Radiation Therapy for Non-Small Cell Lung Cancer ● Chemotherapy for Non-Small Cell Lung Cancer ● Targeted Drug Therapy for Non-Small Cell Lung Cancer ● Immunotherapy for Non-Small Cell Lung Cancer ● Palliative Procedures for Non-Small Cell Lung Cancer Common treatment approaches The treatment options for non-small cell lung cancer (NSCLC) are based mainly on the stage (extent) of the cancer, but other factors, such as a person’s overall health and lung function, as well as certain traits of the cancer itself, are also important. In many cases, more than one of type of treatment is used. ● Treatment Choices for Non-Small Cell Lung Cancer, by Stage Who treats non-small cell lung cancer? You may have different types of doctors on your treatment team, depending on the 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 stage of your cancer and your treatment options. These doctors could include: ● A thoracic surgeon: a doctor who treats diseases of the lungs and chest with surgery ● A radiation oncologist: a doctor who treats cancer with radiation therapy ● A medical oncologist: a doctor who treats cancer with medicines such as chemotherapy, targeted therapy, and immunotherapy ● A pulmonologist: a doctor who specializes in medical treatment of diseases of the lungs Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

Photodynamic Therapy Using Talaporfin Sodium for Local Failure
Journal of Clinical Medicine Article Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience Natsuki Ishida 1 , Satoshi Osawa 2,* , Takahiro Miyazu 1, Masanao Kaneko 1, Satoshi Tamura 1, Shinya Tani 2, Mihoko Yamade 1, Moriya Iwaizumi 3, Yasushi Hamaya 1, Takahisa Furuta 4 and Ken Sugimoto 1 1 First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan; [email protected] (N.I.); [email protected] (T.M.); [email protected] (M.K.); [email protected] (S.T.); [email protected] (M.Y.); [email protected] (Y.H.); [email protected] (K.S.) 2 Department of Endoscopic and Photodynamic Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan; [email protected] 3 Department of Laboratory Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan; [email protected] 4 Center for Clinical Research, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-53-435-2261 Received: 21 April 2020; Accepted: 13 May 2020; Published: 17 May 2020 Abstract: A phase II study of second-generation photodynamic therapy (PDT) using talaporfin sodium has shown excellent treatment results for esophageal cancer with local failure after chemoradiotherapy (CRT) or radiotherapy (RT). However, only a few studies have reported this therapy in clinical practice. -

Cancer Drug Costs for a Month of Treatment at Initial Food
Cancer drug costs for a month of treatment at initial Food and Drug Administration approval Year of FDA Monthly Cost Monthly cost (2013 Generic name Brand name(s) approval (actual $'s) $'s) Vinblastine Velban 1965 $78 $575 Thioguanine, 6-TG Thioguanine Tabloid 1966 $17 $122 Hydroxyurea Hydrea 1967 $14 $97 Cytarabine Cytosar-U, Tarabine PFS 1969 $13 $82 Procarbazine Matulane 1969 $2 $13 Testolactone Teslac 1969 $179 $1,136 Mitotane Lysodren 1970 $134 $801 Plicamycin Mithracin 1970 $50 $299 Mitomycin C Mutamycin 1974 $5 $22 Dacarbazine DTIC-Dome 1975 $29 $125 Lomustine CeeNU 1976 $10 $41 Carmustine BiCNU, BCNU 1977 $33 $127 Tamoxifen citrate Nolvadex 1977 $44 $167 Cisplatin Platinol 1978 $125 $445 Estramustine Emcyt 1981 $420 $1,074 Streptozocin Zanosar 1982 $61 $147 Etoposide, VP-16 Vepesid 1983 $181 $422 Interferon alfa 2a Roferon A 1986 $742 $1,573 Daunorubicin, Daunomycin Cerubidine 1987 $533 $1,090 Doxorubicin Adriamycin 1987 $521 $1,066 Mitoxantrone Novantrone 1987 $477 $976 Ifosfamide IFEX 1988 $1,667 $3,274 Flutamide Eulexin 1989 $213 $399 Altretamine Hexalen 1990 $341 $606 Idarubicin Idamycin 1990 $227 $404 Levamisole Ergamisol 1990 $105 $187 Carboplatin Paraplatin 1991 $860 $1,467 Fludarabine phosphate Fludara 1991 $662 $1,129 Pamidronate Aredia 1991 $507 $865 Pentostatin Nipent 1991 $1,767 $3,015 Aldesleukin Proleukin 1992 $13,503 $22,364 Melphalan Alkeran 1992 $35 $58 Cladribine Leustatin, 2-CdA 1993 $764 $1,229 Asparaginase Elspar 1994 $694 $1,088 Paclitaxel Taxol 1994 $2,614 $4,099 Pegaspargase Oncaspar 1994 $3,006 $4,713 -

North Carolina Medicaid Division of Health Benefits Abbreviated
North Carolina Medicaid Division of Health Benefits Abbreviated Physician Administered Drug Program Catalog •Unless otherwise indicated, the catalog contains procedure codes representing drugs, biologics, devices and vaccines which are only covered for FDA approved indications. •11 digit National Drug Codes (NDCs) are required to be billed along with their corresponding procedure code. Drugs and biologics must be classified as CMS covered outpatient drugs from a labeler/manufacturer participating in the Medicaid Drug Rebate Program (MDRP). •Procedure codes for covered devices and vaccines are not required to be from a rebating labeler/manufacturer as they are not classified as covered outpatient drugs. HCPCS HCPCS Code Description Brand Name Generic Name Code 90291 Cytomegalovirus immune globulin (CMV-IgIV), human, for intravenous use Cytogam® cytomegalovirus immune globulin intravenous, human 90371 Hepatitis B Immune Globulin (Hbig), human, for intramuscular use HyperHep, Nabi-HB hepatitis b immune globulin (human) rabies immune globulin, (human) treated with solvent/detergent, for infiltration and intramuscular 90375 Rabies Immune Globulin (RIg), human, for intramuscular and/or subcutaneous use HyperRAB® S/D, HyperRAB® administration rabies immune globulin, (human) solution for infiltration and intramuscular injection Rabies Immune Globulin, heat-treated (RIg-HT), human, for intramuscular and/or 90376 Imogam® Rabies-HT rabies immune globulin (human), heat treated subcutaneous use 90389 Tetanus Immune Globulin (TIg), human, for intramuscular -

Photodynamic Synergistic Effect of Pheophorbide a and Doxorubicin in Combined Treatment Against Tumoral Cells
cancers Article Photodynamic Synergistic Effect of Pheophorbide a and Doxorubicin in Combined Treatment against Tumoral Cells Rubén Ruiz-González 1, Paula Milán 2, Roger Bresolí-Obach 1, Juan Carlos Stockert 2, Angeles Villanueva 2, Magdalena Cañete 2,* and Santi Nonell 1,* 1 Institut Químic de SarriÒ, Universitat Ramon Llull, Via Augusta 390, 08017 Barcelona, Spain; [email protected] (R.R.-G.); [email protected] (R.B.-O.) 2 Departamento de Biología, Facultad de Ciencias, Universidad Autónoma de Madrid, Darwin 2, 28049 Cantoblanco-Madrid, Spain; [email protected] (P.M.); [email protected] (J.C.S.); [email protected] (A.V.) * Correspondence: [email protected] (M.C.); [email protected] (S.N.); Tel.: +34-914-976-256 (M.C.); +34-932-672-028 (S.N.) Academic Editor: Michael R. Hamblin Received: 8 November 2016; Accepted: 11 February 2017; Published: 17 February 2017 Abstract: A combination of therapies to treat cancer malignancies is at the forefront of research with the aim to reduce drug doses (ultimately side effects) and diminish the possibility of resistance emergence given the multitarget strategy. With this goal in mind, in the present study, we report the combination between the chemotherapeutic drug doxorubicin (DOXO) and the photosensitizing agent pheophorbide a (PhA) to inactivate HeLa cells. Photophysical studies revealed that DOXO can quench the excited states of PhA, detracting from its photosensitizing ability. DOXO can itself photosensitize the production of singlet oxygen; however, this is largely suppressed when bound to DNA. Photodynamic treatments of cells incubated with DOXO and PhA led to different outcomes depending on the concentrations and administration protocols, ranging from antagonistic to synergic for the same concentrations.