Photodynamic Therapy Using Talaporfin Sodium for Local Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -
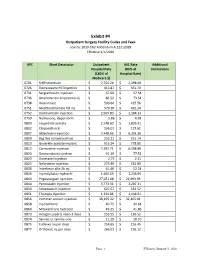
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

NIH Public Access Author Manuscript CA Cancer J Clin
NIH Public Access Author Manuscript CA Cancer J Clin. Author manuscript; available in PMC 2012 July 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: CA Cancer J Clin. 2011 ; 61(4): 250±281. doi:10.3322/caac.20114. PHOTODYNAMIC THERAPY OF CANCER: AN UPDATE Patrizia Agostinis1, Kristian Berg2, Keith A. Cengel3, Thomas H. Foster4, Albert W. Girotti5, Sandra O. Gollnick6, Stephen M. Hahn3, Michael R. Hamblin7,8,9, Asta Juzeniene2, David Kessel10, Mladen Korbelik11, Johan Moan2,12, Pawel Mroz7,8, Dominika Nowis13, Jacques Piette14, Brian C. Wilson15, and Jakub Golab13,16,* 1Department of Molecular Cell Biology, Cell Death Research & Therapy Laboratory, Catholic University of Leuven, B-3000 Leuven, Belgium, [email protected] 2Department of Radiation Biology, Institute for Cancer Research, The Norwegian Radium Hospital, Oslo University Hospital, Montebello, N-0310 Oslo, Norway, [email protected]; [email protected] 3Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA 19004, USA, [email protected]; [email protected] 4Department of Imaging Sciences, University of Rochester, Rochester, NY 14642, USA, [email protected] 5Department of Biochemistry, Medical College of Wisconsin, Milwaukee, WI, 53226-3548, USA, [email protected] 6Department of Cell Stress Biology, Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY, 14263, USA, [email protected] 7Wellman Center for Photomedicine, -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

Oregon Health Authority Division of Medical Assistance Programs Addendum a - Final OPPS Apcs for CY 2012 Effective October 1, 2012
Oregon Health Authority Division of Medical Assistance Programs Addendum A - Final OPPS APCs for CY 2012 Effective October 1, 2012 Relative APC Group Title SI Weight 0001 Level I Photochemotherapy S 0.5042 0002 Fine Needle Biopsy/Aspiration T 1.6115 0003 Bone Marrow Biopsy/Aspiration T 3.5702 0004 Level I Needle Biopsy/ Aspiration Except Bone Marrow T 4.5746 0005 Level II Needle Biopsy/Aspiration Except Bone Marrow T 8.1566 0006 Level I Incision & Drainage T 1.4206 0007 Level II Incision & Drainage T 13.1250 0008 Level III Incision and Drainage T 20.5648 0012 Level I Debridement & Destruction T 0.3878 0013 Level II Debridement & Destruction T 0.8785 0015 Level III Debridement & Destruction T 1.4989 0016 Level IV Debridement & Destruction T 2.7592 0017 Level V Debridement & Destruction T 21.6661 0019 Level I Excision/ Biopsy T 4.4238 0020 Level II Excision/ Biopsy T 8.2746 0021 Level III Excision/ Biopsy T 17.0074 0022 Level IV Excision/ Biopsy T 23.2662 0028 Level I Breast Surgery T 25.5054 0029 Level II Breast Surgery T 33.4070 0030 Level III Breast Surgery T 44.8999 0031 Smoking Cessation Services X 0.2997 0034 Mental Health Services Composite S 2.7295 0035 Vascular Puncture and Minor Diagnostic Procedures X 0.2691 0037 Level IV Needle Biopsy/Aspiration Except Bone Marrow T 15.3499 0039 Level I Implantation of Neurostimulator Generator S 216.7598 0040 Level I Implantation/Revision/Replacement of Neurostimulator Electrodes S 63.7616 0041 Level I Arthroscopy T 29.6568 0042 Level II Arthroscopy T 57.0137 0045 Bone/Joint Manipulation Under -

Original New Drug Applications
New Drugs Reviewed by CPG January 23, 2017 (Original New Drug Applications: FDA) Generic Name Trade Name Indication(s) CPG Action Immune globulin Cuvitru Biologic and In accordance with subcutaneous Immunological the SCA [human] 20% solution Agents/Immune Globulins/ Immune Globulin (Human) subcutaneous. Indicated as replacement for primary humoral immunodeficiency in adult and pediatric patients age two and older. eteplirsen Exondys 51 Central Nervous System In accordance with Agents/Antisense the SCA Oligonucleotides. Treatment of patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. First drug approved for reatment of patients with Duchenne muscular dystrophy. levonorgestrel- Kyleena Endocrine and May prescribe releasing intrauterine Metabolic Agents/sex system 19.5 mg homones/contraceptive Hormones. Prevention of pregnancy for up to five years. **Phentermine Lomaira Central Nervous System In accordance with hydrochloride 8 mg Agents/ the SCA anorexiants/sympathomi metic anorexiants. Short term use weight reduction in adults with **(new formulation at an initial BMI of 30 or lower dosage) more or 27 with at least one weight-related condition. 1 New Drugs January 2017 canaglifozin/ Invokamet XR Endocrine and May prescribe metformin HCL Metabolic Agents/ extended-release antidiabetic agents/antidiabetic combination products. Treatment of adults with type 2 diabetes as an adjunct to diet and exercise. Adalimumab-atto Amjevita Biologic and In accordance with Immunological Agents/ the SCA Immunologic Agents/Immunomodulat ors/Tumor necrosis Factor-Alpha Blockers. Indicated for treatment of adults with: rheumatoid arthritis, psoriatic arthritis,ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis. Also indicated for juvenile idiopathic arthritis in pts age 4 years and older. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Section B Changed Classes/Guidelines Final
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2019 Section B Changed Classes/Guidelines Final Version Date of issue: 24th December 2018 1 A3 FUNCTIONAL GASTRO-INTESTINAL DISORDER DRUGS R2003 A3A PLAIN ANTISPASMODICS AND ANTICHOLINERGICS R1993 Includes all plain synthetic and natural antispasmodics and anticholinergics. A3B Out of use; can be reused. A3C ANTISPASMODIC/ATARACTIC COMBINATIONS This group includes combinations with tranquillisers, meprobamate and/or barbiturates except when they are indicated for disorders of the autonomic nervous system and neurasthenia, in which case they are classified in N5B4. A3D ANTISPASMODIC/ANALGESIC COMBINATIONS R1997 This group includes combinations with analgesics. Products also containing either tranquillisers or barbiturates and analgesics to be also classified in this group. Antispasmodics indicated exclusively for dysmenorrhoea are classified in G2X1. A3E ANTISPASMODICS COMBINED WITH OTHER PRODUCTS r2011 Includes all other combinations not specified in A3C, A3D and A3F. Combinations of antispasmodics and antacids are classified in A2A3; antispasmodics with antiulcerants are classified in A2B9. Combinations of antispasmodics with antiflatulents are classified here. A3F GASTROPROKINETICS r2013 This group includes products used for dyspepsia and gastro-oesophageal reflux. Compounds included are: alizapride, bromopride, cisapride, clebopride, cinitapride, domperidone, levosulpiride, metoclopramide, trimebutine. Prucalopride is classified in A6A9. Combinations of gastroprokinetics with other substances -

Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients with Breast Cancer: Estimates from SEER-Medicare Data
Supplemental online content for: Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients With Breast Cancer: Estimates From SEER-Medicare Data Anne A. Eaton, MS; Camelia S. Sima, MD, MS; and Katherine S. Panageas, DrPH J Natl Compr Canc Netw 2016;14(1):57–65 • eAppendix 1: J-Codes Representing Intravenous Chemotherapy • eAppendix 2: Established Sequential Adjuvant Chemotherapy Regimens for Breast Cancer • eTable 1: Patient Characteristics © JNCCN—Journal of the National Comprehensive Cancer Network | Volume 14 Number 1 | January 2016 Eaton et al - 1 eAppendix 1: J-Codes Representing Intravenous Chemotherapy J-Code Agent J-Code Agent J9000 Injection, doxorubicin HCl, 10 mg J9165 Injection, diethylstilbestrol diphosphate, 250 J9001 Injection, doxorubicin HCl, all lipid mg formulations, 10 mg J9170 Injection, docetaxel, 20 mg J9010 Injection, alemtuzumab, 10 mg J9171 Injection, docetaxel, 1 mg J9015 Injection, aldesleukin, per single use vial J9175 Injection, Elliotts’ B solution, 1 ml J9017 Injection, arsenic trioxide, 1 mg J9178 Injection, epirubicin HCl, 2 mg J9020 Injection, asparaginase, 10,000 units J9179 Injection, eribulin mesylate, 0.1 mg J9025 Injection, azacitidine, 1 mg J9180 Epirubicin HCl, 50 mg J9027 Injection, clofarabine, 1 mg J9181 Injection, etoposide, 10 mg J9031 BCG (intravesical) per instillation J9182 Etoposide, 100 mg J9033 Injection, bendamustine HCl, 1 mg J9185 Injection, fludarabine phosphate, 50 mg J9035 Injection, bevacizumab, 10 mg J9190 Injection, fluorouracil, 500 mg J9040 Injection, -

A Novel 5-Fluorouracil-Resistant Human Esophageal Squamous Cell Carcinoma Cell Line Eca-109/5-FU with Significant Drug Resistance-Related Characteristics
2942 ONCOLOGY REPORTS 37: 2942-2954, 2017 A novel 5-fluorouracil-resistant human esophageal squamous cell carcinoma cell line Eca-109/5-FU with significant drug resistance-related characteristics CHI ZHANG1*, QUNFENG MA2*, YINAN SHI1, XUE LI1, MING WANG1, JUNFENG WANG1, JIANLIN GE1, ZHINAN CHEN3, ZILING WANG1 and HONG JIANG1 1College of Life Science and Bioengineering, School of Science, Beijing Jiaotong University, Haidian, Beijing 100044; 2Department of Thoracic Surgery, Affiliated Hospital of the Academy of Military Medical Sciences, Fengtai, Beijing 100071; 3Cell Engineering Research Center, The Fourth Military Medical University, Xicheng, Xi'an, Shaanxi 710032, P.R. China Received September 1, 2016; Accepted October 31, 2016 DOI: 10.3892/or.2017.5539 Abstract. 5-Fluorouracil (5-FU) is used for the clinical treat- metabolite profile, such as lactate, glutamate, taurine, gluta- ment of esophageal squamous cell carcinomas (ESCCs), yet mine, proline, aspartate, methanol, cystine, glycine and uracil. it also induces chemoresistant cancer cells during treatment, Our results identified that the resistant cell line Eca-109/5-FU which leads to the failure of the therapy. To further explore showed quite different characteristics compared with the the resistance mechanism of 5-FU in ESCC, we established parental Eca-109 cells. The Eca-109/5-FU cell line provides the 5-FU-resistant ESCC cell line Eca-109/5-FU, which was an experimental model for further steps to select chemothera- prepared by the stepwise exposure to increasing 5-FU concen- peutic sensitizers. trations. MTT assay and nude mouse xenograft models were used to test the drug resistance and proliferation of Eca-109 Introduction and Eca-109/5-FU cells in vitro and in vivo. -

I Regulations
23.2.2007 EN Official Journal of the European Union L 56/1 I (Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory) REGULATIONS COUNCIL REGULATION (EC) No 129/2007 of 12 February 2007 providing for duty-free treatment for specified pharmaceutical active ingredients bearing an ‘international non-proprietary name’ (INN) from the World Health Organisation and specified products used for the manufacture of finished pharmaceuticals and amending Annex I to Regulation (EEC) No 2658/87 THE COUNCIL OF THE EUROPEAN UNION, (4) In the course of three such reviews it was concluded that a certain number of additional INNs and intermediates used for production and manufacture of finished pharmaceu- ticals should be granted duty-free treatment, that certain of Having regard to the Treaty establishing the European Commu- these intermediates should be transferred to the list of INNs, nity, and in particular Article 133 thereof, and that the list of specified prefixes and suffixes for salts, esters or hydrates of INNs should be expanded. Having regard to the proposal from the Commission, (5) Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff (1) established the Combined Nomenclature Whereas: (CN) and set out the conventional duty rates of the Common Customs Tariff. (1) In the course of the Uruguay Round negotiations, the Community and a number of countries agreed that duty- (6) Regulation (EEC) No 2658/87 should therefore be amended free treatment should be granted to pharmaceutical accordingly, products falling within the Harmonised System (HS) Chapter 30 and HS headings 2936, 2937, 2939 and 2941 as well as to designated pharmaceutical active HAS ADOPTED THIS REGULATION: ingredients bearing an ‘international non-proprietary name’ (INN) from the World Health Organisation, specified salts, esters or hydrates of such INNs, and designated inter- Article 1 mediates used for the production and manufacture of finished products. -

Standard Oncology Criteria C16154-A
Prior Authorization Criteria Standard Oncology Criteria Policy Number: C16154-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE DUE BEFORE 03/2016 12/2/2020 1/26/2022 HCPCS CODING TYPE OF CRITERIA LAST P&T APPROVAL/VERSION N/A RxPA Q1 2021 20210127C16154-A PRODUCTS AFFECTED: See dosage forms DRUG CLASS: Antineoplastic ROUTE OF ADMINISTRATION: Variable per drug PLACE OF SERVICE: Retail Pharmacy, Specialty Pharmacy, Buy and Bill- please refer to specialty pharmacy list by drug AVAILABLE DOSAGE FORMS: Abraxane (paclitaxel protein-bound) Cabometyx (cabozantinib) Erwinaze (asparaginase) Actimmune (interferon gamma-1b) Calquence (acalbrutinib) Erwinia (chrysantemi) Adriamycin (doxorubicin) Campath (alemtuzumab) Ethyol (amifostine) Adrucil (fluorouracil) Camptosar (irinotecan) Etopophos (etoposide phosphate) Afinitor (everolimus) Caprelsa (vandetanib) Evomela (melphalan) Alecensa (alectinib) Casodex (bicalutamide) Fareston (toremifene) Alimta (pemetrexed disodium) Cerubidine (danorubicin) Farydak (panbinostat) Aliqopa (copanlisib) Clolar (clofarabine) Faslodex (fulvestrant) Alkeran (melphalan) Cometriq (cabozantinib) Femara (letrozole) Alunbrig (brigatinib) Copiktra (duvelisib) Firmagon (degarelix) Arimidex (anastrozole) Cosmegen (dactinomycin) Floxuridine Aromasin (exemestane) Cotellic (cobimetinib) Fludara (fludarbine) Arranon (nelarabine) Cyramza (ramucirumab) Folotyn (pralatrexate) Arzerra (ofatumumab) Cytosar-U (cytarabine) Fusilev (levoleucovorin) Asparlas (calaspargase pegol-mknl Cytoxan (cyclophosphamide)