Oregon Health Authority Division of Medical Assistance Programs Addendum a - Final OPPS Apcs for CY 2012 Effective October 1, 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Photodynamic Therapy with Methyl Aminolevulinate for Primary Nodular Basal Cell Carcinoma: Results of Two Randomized Studies
Clinical trial Photodynamic therapy with methyl aminolevulinate for primary nodular basal cell carcinoma: results of two randomized studies Peter Foley, MBBS, BMedSc, MD, FACD, Michael Freeman, MBBS, FACD, Alan Menter, MB, FAAD, Gregory Siller, MBBS, FACD, Rokea A. El-Azhary, MD, PhD, FAAD, Kurt Gebauer, MBBS, FACD, Nicholas J. Lowe, MD, FAAD, Michael T. Jarratt, MD, FAAD, Dedee F. Murrell, BMBCh, MD, FAAD, Phoebe Rich, MD, FAAD, David M. Pariser, MD, FAAD, Allan R. Oseroff, MD, PhD, FAAD, Ross Barnetson, MD, FRACP, FACD, Christopher Anderson, MBBS, FACD, Steven Kossard, MBBS, FACD, Lawrence E. Gibson, MD, FAAD, and Whitney D. Tope, MPhIL, MD, FAAD From the Department of Medicine Abstract (Dermatology), The University of Background Data suggest that photodynamic therapy using topical methyl aminolevulinate Melbourne, St. Vincent’s Hospital (MAL PDT) may be a noninvasive alternative to excisional surgery for nodular basal cell Melbourne, Fitzroy, Vic., Suite 5 ACH carcinoma (BCC). In the studies described here, we investigated the histologic response, House, Benowa and Department of Dermatology, Princess Alexandra tolerability, and cosmetic outcome with MAL PDT for primary nodular BCC (£ 5 mm in depth). Hospital, Woolloongabba, Qld, Fremantle Methods Two multicenter, randomized, double-blind studies with similar design and Dermatology, Fremantle, WA, and procedures were conducted. After surface debridement and minor tumor debulking, MAL cream Department of Dermatology, St. George 160 mg/g (66 patients with 75 lesions) or placebo cream (65 patients with 75 lesions) was applied Hospital, University of New South Wales, for 3 h, followed by illumination with broad-spectrum red light (75 J/cm2, 570–670 nm). -

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -
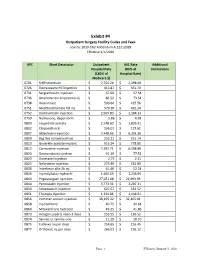
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

Aminolevulinic Acid (ALA) As a Prodrug in Photodynamic Therapy of Cancer
Molecules 2011, 16, 4140-4164; doi:10.3390/molecules16054140 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer Małgorzata Wachowska 1, Angelika Muchowicz 1, Małgorzata Firczuk 1, Magdalena Gabrysiak 1, Magdalena Winiarska 1, Małgorzata Wańczyk 1, Kamil Bojarczuk 1 and Jakub Golab 1,2,* 1 Department of Immunology, Centre of Biostructure Research, Medical University of Warsaw, Banacha 1A F Building, 02-097 Warsaw, Poland 2 Department III, Institute of Physical Chemistry, Polish Academy of Sciences, 01-224 Warsaw, Poland * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel. +48-22-5992199; Fax: +48-22-5992194. Received: 3 February 2011 / Accepted: 3 May 2011 / Published: 19 May 2011 Abstract: Aminolevulinic acid (ALA) is an endogenous metabolite normally formed in the mitochondria from succinyl-CoA and glycine. Conjugation of eight ALA molecules yields protoporphyrin IX (PpIX) and finally leads to formation of heme. Conversion of PpIX to its downstream substrates requires the activity of a rate-limiting enzyme ferrochelatase. When ALA is administered externally the abundantly produced PpIX cannot be quickly converted to its final product - heme by ferrochelatase and therefore accumulates within cells. Since PpIX is a potent photosensitizer this metabolic pathway can be exploited in photodynamic therapy (PDT). This is an already approved therapeutic strategy making ALA one of the most successful prodrugs used in cancer treatment. Key words: 5-aminolevulinic acid; photodynamic therapy; cancer; laser; singlet oxygen 1. Introduction Photodynamic therapy (PDT) is a minimally invasive therapeutic modality used in the management of various cancerous and pre-malignant diseases. -

Pub 100-04 Medicare Claims Processing
Department of Health CMS Manual System & Human Services (DHHS) Pub 100-04 Medicare Claims Centers for Medicare Processing & Medicaid Services (CMS) Transmittal 2128 Date: DECEMBER 29, 2010 Change Request 7275 SUBJECT: January 2011 Update of the Ambulatory Surgical Center (ASC) Payment System I. SUMMARY OF CHANGES: This Recurring Update Notification describes changes to and billing instructions for various payment policies implemented in the January 2011 ASC update. As appropriate, this notification also includes updates to the Healthcare Common Procedure Coding System (HCPCS). EFFECTIVE DATE: January 1, 2011 IMPLEMENTATION DATE: January 3, 2011 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R=REVISED, N=NEW, D=DELETED-Only One Per Row. R/N/D CHAPTER / SECTION / SUBSECTION / TITLE N/A III. FUNDING: For Fiscal Intermediaries (FIs), Regional Home Health Intermediaries (RHHIs): No additional funding will be provided by CMS; Contractor activities are to be carried out within their operating budgets. For Medicare Administrative Contractors (MACs): The Medicare Administrative Contractor is hereby advised that this constitutes technical direction as defined in your contract. CMS does not construe this as a change to the MAC Statement of Work. The contractor is not obligated to incur costs in excess of the amounts allotted in your contract unless and until specifically authorized by the Contracting Officer. -

APPENDICES: a Systematic Review of Photodynamic Therapy in the Treatment of Pre-Cancerous Skin Conditions, Barrett's Oesophagu
Health Technology Assessment 2010; Vol. 14: No.371 Health Technology Assessment 2010; Vol. 14: No. 37 Appendix 5 Pre-cancerous skin scoping Appendix 6 Appendices Go to main text Skin cancer scoping Appendix 7 Barrett’s oesophagus scoping Appendix 8 Oesophageal cancer scoping A systematic review of photodynamic Appendix 9 therapy in the treatment of pre- Lung cancer scoping cancerous skin conditions, Barrett’s Appendix 10 oesophagus and cancers of the biliary Biliary tract cancer scoping Appendix 11 tract, brain, head and neck, lung, Brain cancer scoping oesophagus and skin Appendix 12 Head and neck cancer scoping D Fayter, M Corbett, M Heirs, D Fox Appendix 13 and A Eastwood Actinic keratosis data extraction Appendix 14 Bowen’s disease data extraction Appendix 15 Basal cell carcinoma data extraction Appendix 16 Barrett’s oesophagus data extraction Appendix 17 Oesophageal cancer data extraction Appendix 18 Lung cancer data extraction Appendix 19 Biliary tract cancer data extraction Appendix 20 Brain cancer data extraction Appendix 21 July 2010 Head and neck cancer data extraction 10.3310/hta14370 Health Technology Assessment NIHR HTA programme www.hta.ac.uk HTA How to obtain copies of this and other HTA programme reports An electronic version of this title, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (www.hta.ac.uk). A fully searchable DVD is also available (see below). Printed copies of HTA journal series issues cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our despatch agents. -

Original New Drug Applications
New Drugs Reviewed by CPG January 23, 2017 (Original New Drug Applications: FDA) Generic Name Trade Name Indication(s) CPG Action Immune globulin Cuvitru Biologic and In accordance with subcutaneous Immunological the SCA [human] 20% solution Agents/Immune Globulins/ Immune Globulin (Human) subcutaneous. Indicated as replacement for primary humoral immunodeficiency in adult and pediatric patients age two and older. eteplirsen Exondys 51 Central Nervous System In accordance with Agents/Antisense the SCA Oligonucleotides. Treatment of patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. First drug approved for reatment of patients with Duchenne muscular dystrophy. levonorgestrel- Kyleena Endocrine and May prescribe releasing intrauterine Metabolic Agents/sex system 19.5 mg homones/contraceptive Hormones. Prevention of pregnancy for up to five years. **Phentermine Lomaira Central Nervous System In accordance with hydrochloride 8 mg Agents/ the SCA anorexiants/sympathomi metic anorexiants. Short term use weight reduction in adults with **(new formulation at an initial BMI of 30 or lower dosage) more or 27 with at least one weight-related condition. 1 New Drugs January 2017 canaglifozin/ Invokamet XR Endocrine and May prescribe metformin HCL Metabolic Agents/ extended-release antidiabetic agents/antidiabetic combination products. Treatment of adults with type 2 diabetes as an adjunct to diet and exercise. Adalimumab-atto Amjevita Biologic and In accordance with Immunological Agents/ the SCA Immunologic Agents/Immunomodulat ors/Tumor necrosis Factor-Alpha Blockers. Indicated for treatment of adults with: rheumatoid arthritis, psoriatic arthritis,ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis. Also indicated for juvenile idiopathic arthritis in pts age 4 years and older. -

Methyl-Aminolevulinate-Based-Photodynamic-Therapy-O 2019 Photodiagnosis-And.Pdf
Photodiagnosis and Photodynamic Therapy 26 (2019) 295–299 Contents lists available at ScienceDirect Photodiagnosis and Photodynamic Therapy journal homepage: www.elsevier.com/locate/pdpdt Methyl aminolevulinate-based photodynamic therapy of Bowen´s disease: Observational study of 21 lesions T ⁎ Clara Gómeza, , Marian Cobosb, Enrique Alberdib a Institute of Physical Chemistry Rocasolano, Spanish National Research Council, CSIC, Madrid, Spain b Private clinic of Dr. Alberdi, Madrid, Spain ARTICLE INFO ABSTRACT Keywords: Background: Although surgical removal is the treatment of choice in Bowen's disease (BD), there are cases in Photodynamic therapy which by age, comorbidities, use of anticoagulants, location, cosmetic result, or size, it is preferable to use other Methyl aminolevulinate treatments such as cryotherapy, 5-fluorouracil cream, imiquimod 5% cream or photodynamic therapy (PDT). ’ Bowen s disease Efficacy of PDT in BD is supported by substantial research and clinical data. Objectives: This study aimed to evaluate the long term effectiveness of methyl aminolevulinate-PDT (MAL/PDT) on a wide range of Bowen lesions in different locations and sizes. Methods: Patients diagnosed with BD were treated in 3 sessions with a 4-week interval in between with MAL/ PDT between January 2016 and January 2017 in a private clinic. Clinical response and relevant patient and tumour characteristics were analyzed during the first year after start of the PDT sessions. Results: In total, 21 BD lesions in 18 patients were included in the study. Complete regression (CR) after 3rd PDT session was 87.5% and 100% at the 6-month follow-up. Treatment was well tolerated and local adverse reactions were very scarce. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients with Breast Cancer: Estimates from SEER-Medicare Data
Supplemental online content for: Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients With Breast Cancer: Estimates From SEER-Medicare Data Anne A. Eaton, MS; Camelia S. Sima, MD, MS; and Katherine S. Panageas, DrPH J Natl Compr Canc Netw 2016;14(1):57–65 • eAppendix 1: J-Codes Representing Intravenous Chemotherapy • eAppendix 2: Established Sequential Adjuvant Chemotherapy Regimens for Breast Cancer • eTable 1: Patient Characteristics © JNCCN—Journal of the National Comprehensive Cancer Network | Volume 14 Number 1 | January 2016 Eaton et al - 1 eAppendix 1: J-Codes Representing Intravenous Chemotherapy J-Code Agent J-Code Agent J9000 Injection, doxorubicin HCl, 10 mg J9165 Injection, diethylstilbestrol diphosphate, 250 J9001 Injection, doxorubicin HCl, all lipid mg formulations, 10 mg J9170 Injection, docetaxel, 20 mg J9010 Injection, alemtuzumab, 10 mg J9171 Injection, docetaxel, 1 mg J9015 Injection, aldesleukin, per single use vial J9175 Injection, Elliotts’ B solution, 1 ml J9017 Injection, arsenic trioxide, 1 mg J9178 Injection, epirubicin HCl, 2 mg J9020 Injection, asparaginase, 10,000 units J9179 Injection, eribulin mesylate, 0.1 mg J9025 Injection, azacitidine, 1 mg J9180 Epirubicin HCl, 50 mg J9027 Injection, clofarabine, 1 mg J9181 Injection, etoposide, 10 mg J9031 BCG (intravesical) per instillation J9182 Etoposide, 100 mg J9033 Injection, bendamustine HCl, 1 mg J9185 Injection, fludarabine phosphate, 50 mg J9035 Injection, bevacizumab, 10 mg J9190 Injection, fluorouracil, 500 mg J9040 Injection,