Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies
pharmaceuticals Review Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies Isabella Portugal 1, Sona Jain 1 , Patrícia Severino 1 and Ronny Priefer 2,* 1 Programa de Pós-Graduação em Biotecnologia Industrial, Universidade Tiradentes, Aracaju 49032-490, Brazil; [email protected] (I.P.); [email protected] (S.J.); [email protected] (P.S.) 2 Massachusetts College of Pharmacy and Health Sciences, University, Boston, MA 02115, USA * Correspondence: [email protected] Abstract: Photodynamic therapy is one of the more unique cancer treatment options available in today’s arsenal against this devastating disease. It has historically been explored in cutaneous lesions due to the possibility of focal/specific effects and minimization of adverse events. Advances in drug delivery have mostly been based on biomaterials, such as liposomal and hybrid lipoidal vesicles, nanoemulsions, microneedling, and laser-assisted photosensitizer delivery systems. This review summarizes the most promising approaches to enhancing the photosensitizers’ transdermal delivery efficacy for the photodynamic treatment for cutaneous pre-cancerous lesions and skin cancers. Additionally, discussions on strategies and advantages in these approaches, as well as summarized challenges, perspectives, and translational potential for future applications, will be discussed. Citation: Portugal, I.; Jain, S.; Severino, P.; Priefer, R. Micro- and Keywords: photodynamic therapy; drug delivery; transdermal; cutaneous; cancer Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies. Pharmaceuticals 2021, 1. Introduction 14, 772. https://doi.org/10.3390/ ph14080772 In past decades, clinical demands for the utilization of photosensitizers (PSs) have increased with the advent of photodynamic therapy (PDT). -

Camptothecin Derivatives Camptothecin-Derivate Dérivés De La Camptothécine
(19) TZZ ¥_ _T (11) EP 2 443 125 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 491/14 (2006.01) C07D 491/22 (2006.01) 26.11.2014 Bulletin 2014/48 A61P 35/00 (2006.01) A61K 31/4741 (2006.01) (21) Application number: 10790151.4 (86) International application number: PCT/US2010/038890 (22) Date of filing: 16.06.2010 (87) International publication number: WO 2010/148138 (23.12.2010 Gazette 2010/51) (54) CAMPTOTHECIN DERIVATIVES CAMPTOTHECIN-DERIVATE DÉRIVÉS DE LA CAMPTOTHÉCINE (84) Designated Contracting States: • CAO: "Preparationof 14- nitrocamptothecin...", J. AL AT BE BG CH CY CZ DE DK EE ES FI FR GB CHEM.SOC., PERKIN TRANS. 1, vol. 21, 1996, GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO pages 2629-2632, XP002684965, PL PT RO SE SI SK SM TR • CHENG KEJUN ET AL: "14-azacamptothecin: a potent water-soluble topoisomerase I poison.", (30) Priority: 17.06.2009 US 218043 P JOURNAL OF THE AMERICAN CHEMICAL 16.04.2010 US 325223 P SOCIETY 26 JAN 2005 LNKD- PUBMED: 15656613, vol. 127, no. 3, 26 January 2005 (43) Date of publication of application: (2005-01-26), pages838-839, XP002684966, ISSN: 25.04.2012 Bulletin 2012/17 0002-7863 • VADWAI VIRAL ET AL: "Insilico analysis of (73) Proprietor: Threshold Pharmaceuticals, Inc. homocamptothecin (hCPT) analogues for anti- Redwood City, California 94063 (US) tumour activity", INTERNATIONAL JOURNAL OF BIOINFORMATICS RESEARCH AND (72) Inventors: APPLICATIONS, INDERSCIENCE PUBLISHERS, • CAI, Xiaohong BUCKS, GB, vol. -

Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: a Clinical Case Report
Case Rep Oncol 2018;11:769–776 DOI: 10.1159/000493423 © 2018 The Author(s) Published online: November 27, 2018 Published by S. Karger AG, Basel www.karger.com/cro This article is licensed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (http://www.karger.com/Services/OpenAccessLicense). Usage and distribution for commercial purposes requires written permission. Case Report Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report Lúcio Lara Santosa, b Júlio Oliveiraa Eurico Monteiroa Juliana Santosa Cristina Sarmentoc aPortuguese Institute of Oncology, Porto, Portugal; bExperimental Pathology and Therapeutics Group of Portuguese Institute of Oncology, Porto, Portugal; cUniversity Hospital of São João, Porto, Portugal Keywords Head and neck cancer · Immunotherapy · Immune checkpoint Inhibitor · Photodynamic therapy · Redaporfin Abstract Advanced head and neck squamous cell carcinoma, after locoregional treatment and multiple lines of systemic therapies, represents a great challenge to overcome acquired resistance. The present clinical case illustrates a successful treatment option and is the first to describe the use of photodynamic therapy (PDT) with Redaporfin, followed by immune checkpoint inhibition with an anti-PD1 antibody. This patient presented an extensive tumor in the mouth pavement progressing after surgery, radiotherapy, and multiple lines of systemic treatment. PDT with Redaporfin achieved the destruction of all visible tumor, and the sequential use of an immune checkpoint inhibitor allowed a sustained complete response. This case is an example of the effect of this therapeutic combination and may provide the basis for a new treatment modality. © 2018 The Author(s) Published by S. Karger AG, Basel Lúcio Lara Santos Instituto Português de Oncologia do Porto FG, EPE (IPO-Porto) Rua Dr. -

Re-Visiting Hypersensitivity Reactions to Taxanes: a Comprehensive Review
Clinic Rev Allerg Immunol DOI 10.1007/s12016-014-8416-0 Re-visiting Hypersensitivity Reactions to Taxanes: A Comprehensive Review Matthieu Picard & Mariana C. Castells # Springer Science+Business Media New York 2014 Abstract Taxanes (a class of chemotherapeutic agents) Keywords Taxane . Paclitaxel . Taxol . Docetaxel . are an important cause of hypersensitivity reactions Taxotere . Nab-paclitaxel . Abraxane . Cabazitaxel . (HSRs) in cancer patients. During the last decade, the Chemotherapy . Hypersensitivity . Allergy . Skin test . development of rapid drug desensitization has been key Desensitization . Challenge . Diagnosis . Review . IgE . to allow patients with HSRs to taxanes to be safely re- Complement . Mechanism treated although the mechanisms of these HSRs are not fully understood. Earlier studies suggested that solvents, such as Cremophor EL used to solubilize paclitaxel, Introduction were responsible for HSRs through complement activa- tion, but recent findings have raised the possibility that Hypersensitivity reactions (HSRs) to chemotherapy are in- some of these HSRs are IgE-mediated. Taxane skin creasingly common and represent an important impediment testing, which identifies patients with an IgE-mediated to the care of cancer patients as they may entail serious sensitivity, appears as a promising diagnostic and risk consequences and prevent patients from being treated with stratification tool in the management of patients with the most efficacious agent against their cancer [1]. During the HSRs to taxanes. The management of patients following last decade, different groups have developed rapid drug de- a HSR involves risk stratification and re-exposure could sensitization (RDD) protocols that allow the safe re- be performed either through rapid drug desensitization introduction of a chemotherapeutic agent to which a patient or graded challenge based on the severity of the initial is allergic, and their use have recently been endorsed by the HSR and the skin test result. -

Jevtana® (Cabazitaxel)
AUSTRALIAN PRODUCT INFORMATION – JEVTANA® (CABAZITAXEL) 1 NAME OF THE MEDICINE Cabazitaxel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The concentrated solution for injection contains 60 mg cabazitaxel in 1.5 mL polysorbate 80. Diluent contains 13% w/w ethanol in 4.5 mL water for injections. Excipients of known effect: Diluent contains 13% w/w ethanol. For the full list of excipients, see Section 6.1 List of excipients. 3 PHARMACEUTICAL FORM The concentrated solution for injection is a clear oily yellow to brownish yellow solution. The diluent is a clear, colourless solution. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Jevtana in combination with prednisone or prednisolone is indicated for the treatment of patients with metastatic castration resistant prostate cancer previously treated with a docetaxel containing regimen. 4.2 DOSE AND METHOD OF ADMINISTRATION The use of Jevtana should be confined to units specialised in the administration of cytotoxics and it should only be administered under the supervision of a physician experienced in the use of anticancer chemotherapy. Premedication Premedicate at least 30 minutes prior to each administration of Jevtana with the following intravenous medications to reduce the risk and severity of a hypersensitivity reaction: jevtana-ccdsv10-piv11-10nov20 Page 1 of 26 antihistamine (equivalent to dexchlorpheniramine 5 mg or diphenhydramine 25 mg or equivalent), corticosteroid (dexamethasone 8 mg or equivalent) and with H2 antagonist (ranitidine or equivalent). Antiemetic prophylaxis is recommended and can be given orally or intravenously as needed (see Section 4.4 Special warnings and precautions for use). Recommended Dosage The recommended dose of Jevtana is 20 mg/m2 administered as a 1-hour intravenous infusion every 3 weeks in combination with oral prednisone (or prednisolone) 10 mg administered daily throughout Jevtana treatment. -

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -

Evaluation of Floxuridine Oligonucleotide Conjugates Carrying Potential Enhancers of Cellular Uptake
International Journal of Molecular Sciences Article Evaluation of Floxuridine Oligonucleotide Conjugates Carrying Potential Enhancers of Cellular Uptake Anna Aviñó 1,2,*, Anna Clua 1,2 , Maria José Bleda 1 , Ramon Eritja 1,2,* and Carme Fàbrega 1,2 1 Institute for Advanced Chemistry of Catalonia (IQAC-CSIC), Jordi Girona 18-26, E-08034 Barcelona, Spain; [email protected] (A.C.); [email protected] (M.J.B.); [email protected] (C.F.) 2 Networking Center on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Jordi Girona 18-26, E-08034 Barcelona, Spain * Correspondence: [email protected] (A.A.); [email protected] (R.E.); Tel.: +34-934-006-100 (A.A.) Abstract: Conjugation of small molecules such as lipids or receptor ligands to anti-cancer drugs has been used to improve their pharmacological properties. In this work, we studied the biological effects of several small-molecule enhancers into a short oligonucleotide made of five floxuridine units. Specifically, we studied adding cholesterol, palmitic acid, polyethyleneglycol (PEG 1000), folic acid and triantennary N-acetylgalactosamine (GalNAc) as potential enhancers of cellular uptake. As expected, all these molecules increased the internalization efficiency with different degrees depending on the cell line. The conjugates showed antiproliferative activity due to their metabolic activation by nuclease degradation generating floxuridine monophosphate. The cytotoxicity and apoptosis assays showed an increase in the anti-cancer activity of the conjugates related to the floxuridine oligomer, but this effect did not correlate with the internalization results. Palmitic and folic acid conjugates provide the highest antiproliferative activity without having the highest internalization Citation: Aviñó, A.; Clua, A.; Bleda, results. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

WO 2018/175958 Al 27 September 2018 (27.09.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/175958 Al 27 September 2018 (27.09.2018) W !P O PCT (51) International Patent Classification: A61K 31/53 (2006 .01) A61P 35/00 (2006 .0 1) C07D 251/40 (2006.01) (21) International Application Number: PCT/US20 18/024 134 (22) International Filing Date: 23 March 2018 (23.03.2018) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/476,585 24 March 2017 (24.03.2017) US (71) Applicant: THE REGENTS OF THE UNIVERSITY OF CALIFORNIA [US/US]; 1111 Franklin Street, Twelfth Floor, Oakland, CA 94607-5200 (US). (72) Inventors: NOMURA, Daniel, K.; 4532 Devenport Av enue, Berkeley, CA 94619 (US). ANDERSON, Kimberly, E.; 8 Marchant Court, Kensington, CA 94707 (US). (74) Agent: LEE, Joohee et al; Mintz Levin Cohn Ferris Glovsky And Popeo, P.C., One Financial Center, Boston, MA 021 11 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -
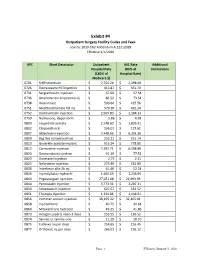
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

Polymer-Drug Conjugate, a Potential Therapeutic to Combat Breast and Lung Cancer
pharmaceutics Review Polymer-Drug Conjugate, a Potential Therapeutic to Combat Breast and Lung Cancer Sibusiso Alven, Xhamla Nqoro , Buhle Buyana and Blessing A. Aderibigbe * Department of Chemistry, University of Fort Hare, Alice Eastern Cape 5700, South Africa; [email protected] (S.A.); [email protected] (X.N.); [email protected] (B.B.) * Correspondence: [email protected] Received: 24 November 2019; Accepted: 30 December 2019; Published: 29 April 2020 Abstract: Cancer is a chronic disease that is responsible for the high death rate, globally. The administration of anticancer drugs is one crucial approach that is employed for the treatment of cancer, although its therapeutic status is not presently satisfactory. The anticancer drugs are limited pharmacologically, resulting from the serious side effects, which could be life-threatening. Polymer drug conjugates, nano-based drug delivery systems can be utilized to protect normal body tissues from the adverse side effects of anticancer drugs and also to overcome drug resistance. They transport therapeutic agents to the target cell/tissue. This review article is based on the therapeutic outcomes of polymer-drug conjugates against breast and lung cancer. Keywords: breast cancer; lung cancer; chemotherapy; polymer-based carriers; polymer-drug conjugates 1. Introduction Cancer is a chronic disease that leads to great mortality around the world and cancer cases are rising continuously [1]. It is the second cause of death worldwide, followed by cardiovascular diseases [2]. It is characterized by an abnormal uncontrolled proliferation of any type of cells in the human body [3]. It is caused by external factors, such as smoking, infectious organisms, pollution, and radiation; it is also caused by internal factors, such as immune conditions, hormones, and genetic mutation [3]. -

Pharmacogenomic Biomarkers in Docetaxel Treatment of Prostate Cancer: from Discovery to Implementation
G C A T T A C G G C A T genes Review Pharmacogenomic Biomarkers in Docetaxel Treatment of Prostate Cancer: From Discovery to Implementation Reka Varnai 1,2, Leena M. Koskinen 3, Laura E. Mäntylä 3, Istvan Szabo 4,5, Liesel M. FitzGerald 6 and Csilla Sipeky 3,* 1 Department of Primary Health Care, University of Pécs, Rákóczi u 2, H-7623 Pécs, Hungary 2 Faculty of Health Sciences, Doctoral School of Health Sciences, University of Pécs, Vörösmarty u 4, H-7621 Pécs, Hungary 3 Institute of Biomedicine, University of Turku, Kiinamyllynkatu 10, FI-20520 Turku, Finland 4 Institute of Sport Sciences and Physical Education, University of Pécs, Ifjúság útja 6, H-7624 Pécs, Hungary 5 Faculty of Sciences, Doctoral School of Biology and Sportbiology, University of Pécs, Ifjúság útja 6, H-7624 Pécs, Hungary 6 Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania 7000, Australia * Correspondence: csilla.sipeky@utu.fi Received: 17 June 2019; Accepted: 5 August 2019; Published: 8 August 2019 Abstract: Prostate cancer is the fifth leading cause of male cancer death worldwide. Although docetaxel chemotherapy has been used for more than fifteen years to treat metastatic castration resistant prostate cancer, the high inter-individual variability of treatment efficacy and toxicity is still not well understood. Since prostate cancer has a high heritability, inherited biomarkers of the genomic signature may be appropriate tools to guide treatment. In this review, we provide an extensive overview and discuss the current state of the art of pharmacogenomic biomarkers modulating docetaxel treatment of prostate cancer. This includes (1) research studies with a focus on germline genomic biomarkers, (2) clinical trials including a range of genetic signatures, and (3) their implementation in treatment guidelines.