Nber Working Paper Series the Impact of New Drug
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Bevacizumab Plus Fotemustine As First-Line Treatment in Metastatic Melanoma Patients: Clinical Activity and Modulation of Angiogenesis and Lymphangiogenesis Factors
Published OnlineFirst October 28, 2010; DOI: 10.1158/1078-0432.CCR-10-2363 Clinical Cancer Cancer Therapy: Clinical Research Bevacizumab plus Fotemustine as First-line Treatment in Metastatic Melanoma Patients: Clinical Activity and Modulation of Angiogenesis and Lymphangiogenesis Factors Michele Del Vecchio1, Roberta Mortarini2, Stefania Canova1, Lorenza Di Guardo1, Nicola Pimpinelli3, Mario R. Sertoli4, Davide Bedognetti4, Paola Queirolo5, Paola Morosini6, Tania Perrone7, Emilio Bajetta1, and Andrea Anichini2 Abstract Purpose: To assess the clinical and biological activity of the association of bevacizumab and fotemustine as first-line treatment in advanced melanoma patients. Experimental Design: Previously untreated, metastatic melanoma patients (n ¼ 20) received bevaci- zumab (at 15 mg/kg every 3 weeks) and fotemustine (100 mg/m2 by intravenous administration on days 1, 8, and 15, repeated after 4 weeks) in a multicenter, single-arm, open-label, phase II study. Primary endpoint was the best overall response rate; other endpoints were toxicity, time to progression (TTP), and overall survival (OS). Serum cytokines, angiogenesis, and lymphangiogenesis factors were monitored by multiplex arrays and by in vitro angiogenesis assays. Effects of fotemustine on melanoma cells, in vitro, on vascular endothelial growth factor (VEGF)-C release and apoptosis were assessed by ELISA and flow cytometry, respectively. Results: One complete response, 2 partial responses (PR), and 10 patients with stable disease were observed. TTP and OS were 8.3 and 20.5 months, respectively. Fourteen patients experienced adverse events of toxicity grade 3–4. Serum VEGF-A levels in evaluated patients (n ¼ 15) and overall serum proangiogenic activity were significantly inhibited. A significant reduction in VEGF-C levels was found in several post-versus pretherapy serum samples. -

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -
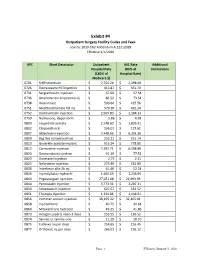
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

Oregon Health Authority Division of Medical Assistance Programs Addendum a - Final OPPS Apcs for CY 2012 Effective October 1, 2012
Oregon Health Authority Division of Medical Assistance Programs Addendum A - Final OPPS APCs for CY 2012 Effective October 1, 2012 Relative APC Group Title SI Weight 0001 Level I Photochemotherapy S 0.5042 0002 Fine Needle Biopsy/Aspiration T 1.6115 0003 Bone Marrow Biopsy/Aspiration T 3.5702 0004 Level I Needle Biopsy/ Aspiration Except Bone Marrow T 4.5746 0005 Level II Needle Biopsy/Aspiration Except Bone Marrow T 8.1566 0006 Level I Incision & Drainage T 1.4206 0007 Level II Incision & Drainage T 13.1250 0008 Level III Incision and Drainage T 20.5648 0012 Level I Debridement & Destruction T 0.3878 0013 Level II Debridement & Destruction T 0.8785 0015 Level III Debridement & Destruction T 1.4989 0016 Level IV Debridement & Destruction T 2.7592 0017 Level V Debridement & Destruction T 21.6661 0019 Level I Excision/ Biopsy T 4.4238 0020 Level II Excision/ Biopsy T 8.2746 0021 Level III Excision/ Biopsy T 17.0074 0022 Level IV Excision/ Biopsy T 23.2662 0028 Level I Breast Surgery T 25.5054 0029 Level II Breast Surgery T 33.4070 0030 Level III Breast Surgery T 44.8999 0031 Smoking Cessation Services X 0.2997 0034 Mental Health Services Composite S 2.7295 0035 Vascular Puncture and Minor Diagnostic Procedures X 0.2691 0037 Level IV Needle Biopsy/Aspiration Except Bone Marrow T 15.3499 0039 Level I Implantation of Neurostimulator Generator S 216.7598 0040 Level I Implantation/Revision/Replacement of Neurostimulator Electrodes S 63.7616 0041 Level I Arthroscopy T 29.6568 0042 Level II Arthroscopy T 57.0137 0045 Bone/Joint Manipulation Under -

Maintenance Therapy in Solid Tumors Marie-Anne Smit, MD, MS,1 and John L
Review Maintenance therapy in solid tumors Marie-Anne Smit, MD, MS,1 and John L. Marshall, MD2 1 Department of Internal Medicine, 2 Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC The concept of maintenance therapy has been well studied in hematologic malignancies, and now, an increasing number of clinical trials explore the role of maintenance therapy in solid cancers. Both biological and lower-intensity chemotherapeutic agents are currently being evaluated as maintenance therapy. However, despite the increase in research in this area, there has not been consensus about the definition and timing of maintenance therapy. In this review, we will focus on continuation maintenance therapy and switch maintenance therapy in patients with metastatic solid tumors who have achieved stable disease, partial response, or complete response after first-line treatment. aintenance therapy is the subject of an apeutic agents, such as capecitabine and oral 5- increased interest in cancer research. fluorouracil (5-FU), are currently being evaluated as MIn contrast to conventional chemo- maintenance therapy. therapy that aims to kill as many cancer cells as Despite the increase in research in this area, possible, the goal of treatment with maintenance there is no consensus on the definition and timing therapy is to sustain a stable tumor mass, reduce of maintenance therapy. The term maintenance cancer-related symptoms, and prolong the time to therapy is used in a variety of treatment situations, progression and the related symptoms. A thera- such as prolonged first-line therapy and less- peutic strategy that is explicitly designed to main- intense or different therapy given after first-line tain a stable, tolerable tumor volume could in- therapy. -

An Analysis of Toxicity and Response Outcomes from Dose
Brock et al. BMC Cancer (2021) 21:777 https://doi.org/10.1186/s12885-021-08440-0 RESEARCH ARTICLE Open Access Is more better? An analysis of toxicity and response outcomes from dose-finding clinical trials in cancer Kristian Brock1* ,VictoriaHomer1, Gurjinder Soul2, Claire Potter1,CodyChiuzan3 and Shing Lee3 Abstract Background: The overwhelming majority of dose-escalation clinical trials use methods that seek a maximum tolerable dose, including rule-based methods like the 3+3, and model-based methods like CRM and EWOC. These methods assume that the incidences of efficacy and toxicity always increase as dose is increased. This assumption is widely accepted with cytotoxic therapies. In recent decades, however, the search for novel cancer treatments has broadened, increasingly focusing on inhibitors and antibodies. The rationale that higher doses are always associated with superior efficacy is less clear for these types of therapies. Methods: We extracted dose-level efficacy and toxicity outcomes from 115 manuscripts reporting dose-finding clinical trials in cancer between 2008 and 2014. We analysed the outcomes from each manuscript using flexible non-linear regression models to investigate the evidence supporting the monotonic efficacy and toxicity assumptions. Results: We found that the monotonic toxicity assumption was well-supported across most treatment classes and disease areas. In contrast, we found very little evidence supporting the monotonic efficacy assumption. Conclusions: Our conclusion is that dose-escalation trials routinely use methods whose assumptions are violated by the outcomes observed. As a consequence, dose-finding trials risk recommending unjustifiably high doses that may be harmful to patients. We recommend that trialists consider experimental designs that allow toxicity and efficacy outcomes to jointly determine the doses given to patients and recommended for further study. -

Original New Drug Applications
New Drugs Reviewed by CPG January 23, 2017 (Original New Drug Applications: FDA) Generic Name Trade Name Indication(s) CPG Action Immune globulin Cuvitru Biologic and In accordance with subcutaneous Immunological the SCA [human] 20% solution Agents/Immune Globulins/ Immune Globulin (Human) subcutaneous. Indicated as replacement for primary humoral immunodeficiency in adult and pediatric patients age two and older. eteplirsen Exondys 51 Central Nervous System In accordance with Agents/Antisense the SCA Oligonucleotides. Treatment of patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. First drug approved for reatment of patients with Duchenne muscular dystrophy. levonorgestrel- Kyleena Endocrine and May prescribe releasing intrauterine Metabolic Agents/sex system 19.5 mg homones/contraceptive Hormones. Prevention of pregnancy for up to five years. **Phentermine Lomaira Central Nervous System In accordance with hydrochloride 8 mg Agents/ the SCA anorexiants/sympathomi metic anorexiants. Short term use weight reduction in adults with **(new formulation at an initial BMI of 30 or lower dosage) more or 27 with at least one weight-related condition. 1 New Drugs January 2017 canaglifozin/ Invokamet XR Endocrine and May prescribe metformin HCL Metabolic Agents/ extended-release antidiabetic agents/antidiabetic combination products. Treatment of adults with type 2 diabetes as an adjunct to diet and exercise. Adalimumab-atto Amjevita Biologic and In accordance with Immunological Agents/ the SCA Immunologic Agents/Immunomodulat ors/Tumor necrosis Factor-Alpha Blockers. Indicated for treatment of adults with: rheumatoid arthritis, psoriatic arthritis,ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis. Also indicated for juvenile idiopathic arthritis in pts age 4 years and older. -

Hyperthermic Isolated Hepatic Perfusion Using Melphalan for Patients with Ocular Melanoma Metastatic to Liver
Vol. 9, 6343–6349, December 15, 2003 Clinical Cancer Research 6343 Hyperthermic Isolated Hepatic Perfusion Using Melphalan for Patients with Ocular Melanoma Metastatic to Liver H. Richard Alexander, Jr.,1 Steven K. Libutti,1 survival after disease progression is short. On the basis of a James F. Pingpank,1 Seth M. Steinberg,2 pattern of tumor progression predominantly in liver, con- David L. Bartlett,1 Cynthia Helsabeck,1 and tinued clinical evaluation of hepatic directed therapy in this patient population is justified. Tatiana Beresneva1 1Surgical Metabolism Section, Surgery Branch, Center for Cancer Research and 2Biostatistics and Data Management Section, Center for INTRODUCTION Cancer Research, National Cancer Institute, Bethesda, Maryland There are ϳ54,200 new cases of malignant melanoma diagnosed annually in the United States (1), of which 3–6% are ABSTRACT primary ocular melanoma representing up to 4,000 new cases/ Purpose: Liver metastases are the sole or life-limiting year (2). Metastatic disease occurs in 40–60% of patients with component of disease in the majority of patients with ocular ocular melanoma (3), and unresectable hepatic metastases rep- melanoma who recur. Because median survival after diag- resent the life-limiting component of progressive disease in nosis of liver metastases is short and no satisfactory treat- ϳ80% of patients with ocular melanoma in this setting (3–5). ment options exist, we have conducted clinical trials evalu- Even with aggressive treatment, the median survival after liver ating isolated hepatic perfusion (IHP) for patients afflicted metastases from ocular melanoma are diagnosed is between 2 with this condition. and 7 months and 1-year survival is ϳ10% (5). -

Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients with Breast Cancer: Estimates from SEER-Medicare Data
Supplemental online content for: Prevalence and Safety of Off-Label Use of Chemotherapeutic Agents in Older Patients With Breast Cancer: Estimates From SEER-Medicare Data Anne A. Eaton, MS; Camelia S. Sima, MD, MS; and Katherine S. Panageas, DrPH J Natl Compr Canc Netw 2016;14(1):57–65 • eAppendix 1: J-Codes Representing Intravenous Chemotherapy • eAppendix 2: Established Sequential Adjuvant Chemotherapy Regimens for Breast Cancer • eTable 1: Patient Characteristics © JNCCN—Journal of the National Comprehensive Cancer Network | Volume 14 Number 1 | January 2016 Eaton et al - 1 eAppendix 1: J-Codes Representing Intravenous Chemotherapy J-Code Agent J-Code Agent J9000 Injection, doxorubicin HCl, 10 mg J9165 Injection, diethylstilbestrol diphosphate, 250 J9001 Injection, doxorubicin HCl, all lipid mg formulations, 10 mg J9170 Injection, docetaxel, 20 mg J9010 Injection, alemtuzumab, 10 mg J9171 Injection, docetaxel, 1 mg J9015 Injection, aldesleukin, per single use vial J9175 Injection, Elliotts’ B solution, 1 ml J9017 Injection, arsenic trioxide, 1 mg J9178 Injection, epirubicin HCl, 2 mg J9020 Injection, asparaginase, 10,000 units J9179 Injection, eribulin mesylate, 0.1 mg J9025 Injection, azacitidine, 1 mg J9180 Epirubicin HCl, 50 mg J9027 Injection, clofarabine, 1 mg J9181 Injection, etoposide, 10 mg J9031 BCG (intravesical) per instillation J9182 Etoposide, 100 mg J9033 Injection, bendamustine HCl, 1 mg J9185 Injection, fludarabine phosphate, 50 mg J9035 Injection, bevacizumab, 10 mg J9190 Injection, fluorouracil, 500 mg J9040 Injection, -

II. Recurring Tumors
Review Article Open Access J Surg Volume 7 Issue 1 - November 2017 Copyright © All rights are reserved by Alain L Fymat DOI: 10.19080/OAJS.2017.07.555703 Surgical and Non-Surgical Management and Treatment of Glioblastoma: II. Recurring Tumors Alain L Fymat* International Institute of Medicine & Science, USA Submission: October 28, 2017; Published: November 16, 2017 *Corresponding author: Alain L Fymat, International Institute of Medicine and Science, California, USA, Tel: ; Email: Abstract Glioblastoma (also known as glioblastoma multiform) is the most common primary brain tumor in adults. It remains an unmet need in oncology. Complementing an earlier discussion of primary and secondary tumors in both cases of monotherapies and combined therapies, Clinical trials and other reported practices will also be discussed and summarized. Regarding chemotherapy, whereas it has historically surgery,provided conformal little durable radiotherapy, benefit with boron tumors neutron recurring therapy, within intensity several modulated months, forproton brain beam tumors, therapy, the access antiangiogenic is hindered therapy, or even alternating forbidden electric by the presence of the brain protective barriers, chiefly the blood brain barrier. More effective therapies involving other options are required including immunotherapy, adjuvant therapy, gene therapy, stem cell therapy, and intra-nasal drug delivery. field therapy, ...without neglecting palliative therapies. Research conducted in these and other options is also reviewed to include microRNA, -

Neoplasia Treatment Compositions Containing Antineoplastic Agent and Side-Effect Reducing Protective Agent
~" ' MM II II II INI MM II III 1 1 II I II J European Patent Office _ _ _ © Publication number: 0 393 575 B1 Office_„. europeen des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 16.03.94 © Int. CI.5: A61 K 45/06, A61 K 31/785, //(A61K31/785,31:71 ,31:70) © Application number: 90107246.2 @ Date of filing: 17.04.90 © Neoplasia treatment compositions containing antineoplastic agent and side-effect reducing protective agent. ® Priority: 17.04.89 US 339503 © Proprietor: G.D. Searle & Co. P.O. Box 5110 @ Date of publication of application: Chicago Illinois 60680(US) 24.10.90 Bulletin 90/43 @ Inventor: Bach, Ardalan © Publication of the grant of the patent: 3570 Matheson 16.03.94 Bulletin 94/11 Coconut Grove Florida 33133(US) Inventor: Shanahan, William R.Jr © Designated Contracting States: 331 Pumpkin Hill Road AT BE CH DE DK ES FR GB GR IT LI LU NL SE Ledyard, Connecticut 06339(US) © References cited: GB-A- 2 040 951 © Representative: Bell, Hans Chr., Dr. et al BEIL, WOLFF & BEIL, CHEMICAL ABSTRACTS, vol. 103, no. 12, 23rd Rechtsanwalte, September 1985, page 323, abstract no. Postfach 80 01 40 92828x, Columbus, Ohio, US; D-65901 Frankfurt (DE) 00 lo iv lo oo o> oo Note: Within nine months from the publication of the mention of the grant of the European patent, any person ® may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition CL shall be filed in a written reasoned statement. -

Other Alkylating Agents
Anticancer agents The Alkylating agents The alkylating agents are the oldest group of cytostatic drugs. The first of them – mechlorethamine was introduced into therapy in 1949. The alkylating agents can contain one or two alkylating groups. In the body the alkylating agents may react with DNA, RNA and proteins, although their reaction with DNA is believed to be the most important. 1 • The primary target of DNA coross-linking agents is the actively dividing DNA molecule. • The DNA cross-linkers are all extremly reactive nucleophilic structures (+). • When encountered, the nucleophilic groups on various DNA bases (particularly, but not exclusively, the N7 of guaninę) readily attack the electrophilic drud, resulting in irreversible alkylation or complexation of the DNA base. • Some DNA alkylating agents, such as the nitrogen mustards and nitrosoureas, are bifunctional, meaning that one molecule of the drug can bind two distinct DNA bases. 2 • Most commonly, the alkylated bases are on different DNA molecules, and intrastrand DNA cross-linking through two guaninę N7 atoms results. • The DNA alkylating antineoplastics are not cel cycle specific, but they are more toxic to cells in the late G1 or S phases of the cycle. • This is a time when DNA unwinding and exposing its nucleotides, increasing the Chance that vulnerable DNA functional groups will encounter the nucleophilic antineoplastic drug and launch the nucleophilic attack that leads to its own destruction. • The DNA alkylators have a great capacity for inducing toth mutagenesis and carcinogenesis, they can promote caner in addition to treating it. 3 • Organometallic antineoplastics – Platinum coordination complexes, also cross-link DNA, and many do so by binding to adjacent guanine nucleotides, called disguanosine dinucleotides, on the single strand of DNA.