List of Off-Patent, Off-Exclusivity Drugs Without an Approved Generic
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

LYSODREN Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS------------------------ These highlights do not include all the information needed to use • Central Nervous System (CNS) Toxicity: Plasma concentrations LYSODREN safely and effectively. See full prescribing information for exceeding 20 mcg/mL are associated with a greater incidence of toxicity. LYSODREN. (5.2) • Adrenal Insufficiency: Institute steroid replacement as clinically LYSODREN® (mitotane) tablets, for oral use indicated. Measure free cortisol and corticotropin (ACTH) levels to Initial U.S. Approval: 1970 achieve optimal steroid replacement. (5.3) • Embryo-Fetal Toxicity: Can cause fetal harm. Advise women of WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR reproductive potential of the potential risk to a fetus and use of effective SEVERE TRAUMA contraception. (5.4, 8.1, 8.3) See full prescribing information for complete boxed warning. • Ovarian Macrocysts in Premenopausal Women: Advise women to seek medical advice if they experience gynecological symptoms such as In patients taking LYSODREN, adrenal crisis occurs in the setting of vaginal bleeding and/or pelvic pain. (5.5) shock or severe trauma and response to shock is impaired. Administer hydrocortisone, monitor for escalating signs of shock and discontinue -------------------------------ADVERSE REACTIONS------------------------------ LYSODREN until recovery. (2.2, 5.1) Common adverse reactions (≥15%) include: anorexia, nausea, vomiting and diarrhea; depression, dizziness or vertigo; and rash. (6) ---------------------------INDICATIONS AND USAGE---------------------------- LYSODREN is an adrenal cytotoxic agent indicated for the treatment of To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers inoperable, functional or nonfunctional, adrenal cortical carcinoma. (1) Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

EDP & Mitotane Protocol
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHA HANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical carcinoma ECOG performance status of 0 - 2 All possible tumour tissues should be surgically removed from large metastatic masses before mitotane administration is instituted. This is necessary to minimise the possibility of infarction and haemorrhage in the tumour due to a rapid cytotoxic effect of mitotane Exclusion criteria: Previous complete cumulative doses of anthracyclines Decompensated heart failure (ejection fraction <50%) Unstable angina, uncontrolled cardiac arrhythmias, MI or revascularisation procedure within last 6 months Dosage: Drug Dosage Route Frequency Etoposide 100mg/m2 IV Daily on days 2, 3 and 4 of each cycle Doxorubicin 40mg/m2 IV Once only on day 1 of each cycle Cisplatin 40mg/m2 IV Daily on days 3 and 4 of each cycle Variable, max Continuous, started a week prior to IV Mitotane PO 6g daily chemotherapy if not already taking With cycles repeated at 28 day intervals until disease progression Supportive treatments: Domperidone 10mg oral tablets maximum 3 times a day or as required Issue Date: May 2017 Page 1 of 8 Protocol reference: MPHAHANEDP 1 Authorised by: Drugs & Therapeutics Author: Helen Flint Committee & Dr D Husband Version No: 1.0 THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST Extravasation risk: Doxorubicin is a vesicant and must be observed during administration. Follow the procedure for anthracycline extravasation. Erythematous streaking (a histamine release phenomenon) along the vein proximal to the site of injection has been reported, and must be differentiated from an extravasation event. -

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -

List of Approved Ndas for Biological Products That Were Deemed to Be Blas on March 23, 2020
List of Approved NDAs for Biological Products That Were Deemed to be BLAs on March 23, 2020 On March 23, 2020, an approved application for a biological product under section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) was deemed to be a license for the biological product under section 351 of the Public Health Service Act (PHS Act) (see section 7002(e)(4)(A) of the Biologics Price Competition and Innovation Act of 2009). To enhance transparency and facilitate planning for the March 23, 2020, transition date, FDA compiled a preliminary list of approved applications for biological products under the FD&C Act that were listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book) and that would be affected by this transition provision. FDA posted this list on the FDA website in December 2018, and periodically updated this list before the March 23, 2020, transition date. The September 2019 update to this preliminary list added certain administratively closed applications related to approved applications for biological products that were on the December 2018 version of this list. The January 2020 update to the preliminary list reflected a change to the definition of “biological product” made by the Further Consolidated Appropriations Act, 2020, which was enacted on December 20, 2019. Section 605 of this Act further amended the definition of a “biological product” in section 351(i) of the PHS Act to remove the parenthetical “(except any chemically synthesized polypeptide)” from the statutory category of “protein.” FDA has provided below a list of each approved application for a biological product under the FD&C Act that was deemed to be a license (i.e., an approved biologics license application (BLA)) for the biological product on March 23, 2020. -

30 Day Change Notice Effective Date
30 Day Change Notice Effective Date: January 1st, 2021 NEW PREFERRED DRUGS THERAPEUTIC CLASS NO PA REQUIRED PREFERRED Central Nervous System (CNS) Agents: Anticonvulsants Clobazam (Generic of Onfi) Central Nervous System (CNS) Agents: Multiple Aubagio EndocrineSclerosis Agents: Osteoporosis-Bone Ossification Forteo Enhancers Gastrointestinal Agents: Anti-Emetics Bonjesta Genitourinary Agents: Benign Prostatic Hyperplasia Alfuzosin (Generic of Uroxatral) Dutasteride (Generic of Avodart) Genitourinary Agents: Electrolyte Depleter Agents Sevelamer (Generic of Renagel and Renvela) Infectious Disease Agents: Antibiotics-Macrolides Eryped Infectious Disease Agents: Antivirals-HIV Atazanavir Sulfate Oral Powder (Generic of Reyataz) Tivicay PD Infectious Disease Agents: Antibiotics-Tetracyclines Vibramycin Suspension (no PA Required for age 12 or under) Ophthalmic Agents: Antibiotics and Antibiotic -Steroid Neomycin/Polymyxin/Bacitracin/Hydrocortisone Ointment Combination Drops and Ointments Ophthalmic Agents: Glaucoma Agents Dorzolamide/Timolol (Generic of Cosopt PF) NEW CLINICAL PA REQUIRED “PREFERRED” DRUGS THERAPEUTIC CLASS CLINICAL PA REQUIRED PREFERRED Blood Formation, Coagulation, and Thrombosis Agents: Corifact Hemophilia Factors Immunomodulator Agents for Systemic Inflammatory Taltz Disease Immunomodulator Agents for Systemic Inflammatory Xeljanz 5mg Disease NEW STEP THERAPY REQUIRED “PREFERRED” THERAPEUTIC CLASS STEP THERAPY REQUIRED “PREFERRED” Central nervous System (CNS) Agents: Anti-Migraine, Aimovig Prophylaxis Treatment Ajovy -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes). -

Use of Fluorescence to Guide Resection Or Biopsy of Primary Brain Tumors and Brain Metastases
Neurosurg Focus 36 (2):E10, 2014 ©AANS, 2014 Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases *SERGE MARBACHER, M.D., M.SC.,1,5 ELISABETH KLINGER, M.D.,2 LUCIA SCHWYZER, M.D.,1,5 INGEBORG FISCHER, M.D.,3 EDIN NEVZATI, M.D.,1 MICHAEL DIEPERS, M.D.,2,5 ULRICH ROELCKE, M.D.,4,5 ALI-REZA FATHI, M.D.,1,5 DANIEL COLUCCIA, M.D.,1,5 AND JAVIER FANDINO, M.D.1,5 Departments of 1Neurosurgery, 2Neuroradiology, 3Pathology, and 4Neurology, and 5Brain Tumor Center, Kantonsspital Aarau, Aarau, Switzerland Object. The accurate discrimination between tumor and normal tissue is crucial for determining how much to resect and therefore for the clinical outcome of patients with brain tumors. In recent years, guidance with 5-aminolev- ulinic acid (5-ALA)–induced intraoperative fluorescence has proven to be a useful surgical adjunct for gross-total resection of high-grade gliomas. The clinical utility of 5-ALA in resection of brain tumors other than glioblastomas has not yet been established. The authors assessed the frequency of positive 5-ALA fluorescence in a cohort of pa- tients with primary brain tumors and metastases. Methods. The authors conducted a single-center retrospective analysis of 531 patients with intracranial tumors treated by 5-ALA–guided resection or biopsy. They analyzed patient characteristics, preoperative and postoperative liver function test results, intraoperative tumor fluorescence, and histological data. They also screened discharge summaries for clinical adverse effects resulting from the administration of 5-ALA. Intraoperative qualitative 5-ALA fluorescence (none, mild, moderate, and strong) was documented by the surgeon and dichotomized into negative and positive fluorescence. -

Promising Findings for SCC in Organ Transplant Recipients Repeated PDT Treatments May Reduce the Incidence of Sccs in This High-Risk Population
Oncology Watch Cyclic Photodynamic Therapy: Promising Findings for SCC in Organ Transplant Recipients Repeated PDT treatments may reduce the incidence of SCCs in this high-risk population. By Jonathan Wolfe, MD hotodynamic therapy with 5-aminolevulinic acid SOTRs typically undergo chemoprophylaxis with sys- (ALA, Levulan, DUSA Pharmaceuticals) has temic retinoids, although there have been few ran- become established as a safe and effective option domized controlled trials to quantify their benefit. Pfor the management of AKs (see the January edi- Acitretin is probably the most frequently used agent,3 tion, available at PracticalDermatology.com). The drug but isotretinoin and etretinate are also used, and there is indicated, along with blue light application, for the is anecdotal evidence to support the use of treatment of minimally to moderately thick actinic bexarotene.4 Of note, rebound flares have been associ- keratoses of the face or scalp. However, there has ated with discontinuation of retinoids, leading some to been increasing interest in the role of PDT to manage advocate chemoprevention as a lifelong therapy.3 other types of skin cancers. A recent study shows that the procedure may be effective for reducing the inci- An Emerging Option dence of squamous cell carcinoma (SCC) in solid Given the concerns about high rates of NMSCs in organ transplant recipients (SOTR). transplant recipients, there is interest in identifying optimal treatment and prevention strategies. While Skin Cancer and SOTRs retinoid chemoprophylaxis is an important and effec- Compared to the general population, solid organ trans- tive option, additional interventions are welcome. plant recipients are at higher risk of skin cancer, with Recent findings suggest a role for cyclic 5-ALA PDT up to a 100-fold estimated increase in the relative risk therapy.5 Twelve high-risk SOTRs received cyclic PDT of squamous cell carcinoma (SCC) compared to the treatments at four- to eight-week intervals for two non-transplanted population. -

Incidence of Differentiation Syndrome Associated with Treatment
Journal of Clinical Medicine Review Incidence of Differentiation Syndrome Associated with Treatment Regimens in Acute Myeloid Leukemia: A Systematic Review of the Literature Lucia Gasparovic 1, Stefan Weiler 1,2, Lukas Higi 1 and Andrea M. Burden 1,* 1 Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland; [email protected] (L.G.); [email protected] (S.W.); [email protected] (L.H.) 2 National Poisons Information Centre, Tox Info Suisse, Associated Institute of the University of Zurich, 8032 Zurich, Switzerland * Correspondence: [email protected]; Tel.: +41-76-685-22-56 Received: 30 August 2020; Accepted: 14 October 2020; Published: 18 October 2020 Abstract: Differentiation syndrome (DS) is a potentially fatal adverse drug reaction caused by the so-called differentiating agents such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), used for remission induction in the treatment of the M3 subtype of acute myeloid leukemia (AML), acute promyelocytic leukemia (APL). However, recent DS reports in trials of isocitrate dehydrogenase (IDH)-inhibitor drugs in patients with IDH-mutated AML have raised concerns. Given the limited knowledge of the incidence of DS with differentiating agents, we conducted a systematic literature review of clinical trials with reports of DS to provide a comprehensive overview of the medications associated with DS. In particular, we focused on the incidence of DS reported among the IDH-inhibitors, compared to existing ATRA and ATO therapies. We identified 44 published articles, encompassing 39 clinical trials, including 6949 patients. Overall, the cumulative incidence of DS across all treatment regimens was 17.7%. -
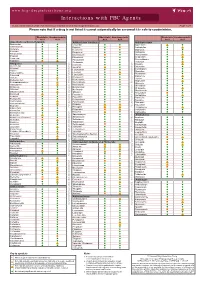
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

Nurse-Led Drug Monitoring Clinic Protocol for the Use of Systemic Therapies in Dermatology for Patients
Group arrangements: Salford Royal NHS Foundation Trust (SRFT) Pennine Acute Hospitals NHS Trust (PAT) Nurse-led drug monitoring clinic protocol for the use of systemic therapies in dermatology for patients with inflammatory dermatoses Lead Author: Dawn Lavery Dermatology Advanced Nurse Practitioner Additional author(s) N/A Division/ Department:: Dermatology, Clinical Support and Tertiary Medicine Applies to: (Please delete) Salford Royal Care Organisation Approving Committee Dermatology clinical governance committee Salford Royal Date approved: 13 February 2019 Expiry date: February 2022 Contents Contents Section Page Document summary sheet 1 Overview 2 2 Scope & Associated Documents 2 3 Background 3 4 What is new in this version? 3 5 Policy 4 Drugs monitored by nurses 4 Acitretin 7 Alitretinoin Toctino 11 Apremilast 22 Azathioprine 26 Ciclosporin 29 Dapsone 34 Fumaric Acid Esters – Fumaderm and Skilarence 36 Hydroxycarbamide 39 Hydroxychloroquine 43 Methotrexate 50 Mycophenolate moefetil 57 Nurse-led drug monitoring clinic protocol for the use of systemic therapies in dermatology for patients with inflammatory dermatoses Reference Number GSCDerm01(13) Version 3 Issue Date: 11/06/2019 Page 1 of 77 It is your responsibility to check on the intranet that this printed copy is the latest version Standards 67 6 Roles and responsibilities 67 7 Monitoring document effectiveness 67 8 Abbreviations and definitions 68 9 References 68 10 Appendices N/A 11 Document Control Information 71 12 Equality Impact Assessment (EqIA) screening tool 73 Group arrangements: Salford Royal NHS Foundation Trust (SRFT) Pennine Acute Hospitals NHS Trust (PAT) 1. Overview (What is this policy about?) The dermatology directorate specialist nurses are responsible for ensuring prescribing and monitoring for patients under their care, is in accordance with this protocol. -

WHO Model List of Essential Medicines
WHO Model List of Essential Medicines 15th list, March 2007 Status of this document This is a reprint of the text on the WHO Medicines web site http://www.who.int/medicines/publications/essentialmedicines/en/index.html 15th edition Essential Medicines WHO Model List (revised March 2007) Explanatory Notes The core list presents a list of minimum medicine needs for a basic health care system, listing the most efficacious, safe and cost‐effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost‐effective treatment. The complementary list presents essential medicines for priority diseases, for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed. In case of doubt medicines may also be listed as complementary on the basis of consistent higher costs or less attractive cost‐effectiveness in a variety of settings. The square box symbol () is primarily intended to indicate similar clinical performance within a pharmacological class. The listed medicine should be the example of the class for which there is the best evidence for effectiveness and safety. In some cases, this may be the first medicine that is licensed for marketing; in other instances, subsequently licensed compounds may be safer or more effective. Where there is no difference in terms of efficacy and safety data, the listed medicine should be the one that is generally available at the lowest price, based on international drug price information sources. Therapeutic equivalence is only indicated on the basis of reviews of efficacy and safety and when consistent with WHO clinical guidelines.