Promising Findings for SCC in Organ Transplant Recipients Repeated PDT Treatments May Reduce the Incidence of Sccs in This High-Risk Population
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Incidence of Differentiation Syndrome Associated with Treatment
Journal of Clinical Medicine Review Incidence of Differentiation Syndrome Associated with Treatment Regimens in Acute Myeloid Leukemia: A Systematic Review of the Literature Lucia Gasparovic 1, Stefan Weiler 1,2, Lukas Higi 1 and Andrea M. Burden 1,* 1 Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland; [email protected] (L.G.); [email protected] (S.W.); [email protected] (L.H.) 2 National Poisons Information Centre, Tox Info Suisse, Associated Institute of the University of Zurich, 8032 Zurich, Switzerland * Correspondence: [email protected]; Tel.: +41-76-685-22-56 Received: 30 August 2020; Accepted: 14 October 2020; Published: 18 October 2020 Abstract: Differentiation syndrome (DS) is a potentially fatal adverse drug reaction caused by the so-called differentiating agents such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), used for remission induction in the treatment of the M3 subtype of acute myeloid leukemia (AML), acute promyelocytic leukemia (APL). However, recent DS reports in trials of isocitrate dehydrogenase (IDH)-inhibitor drugs in patients with IDH-mutated AML have raised concerns. Given the limited knowledge of the incidence of DS with differentiating agents, we conducted a systematic literature review of clinical trials with reports of DS to provide a comprehensive overview of the medications associated with DS. In particular, we focused on the incidence of DS reported among the IDH-inhibitors, compared to existing ATRA and ATO therapies. We identified 44 published articles, encompassing 39 clinical trials, including 6949 patients. Overall, the cumulative incidence of DS across all treatment regimens was 17.7%. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Resimmune, an Anti-Cd3ε Recombinant Immunotoxin, Induces
Non-Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Resimmune, an anti-CD3 recombinant immunotoxin, induces durable remissions in patients wiεth cutaneous T-cell lymphoma Arthur E. Frankel, 1 Jung H. Woo, 2 Chul Ahn, 1 Francine M. Foss, 3 Madeleine Duvic, 4 Paul H. Neville, 5 and David M. Neville 5 1University of Texas Southwestern Medical Center, Dallas, TX; 2Baylor Scott & White Health, Temple, TX; 3Yale University School of Medi - cine, New Haven, CT; 4The University of Texas MD Anderson Cancer Center, Houston, TX; and 5Angimmune, LLC, Bethesda, MD, USA ©2015 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.123711 Manuscript received on January 20, 2015. Manuscript accepted on March 18, 2015. Correspondence: [email protected] Supplemental Material Administration-- The study was performed under the sponsorship of Angimmune, LLC, registered in clinical trials.gov as NCT00611208, and approved by Institutional Review Boards at the participating institutions. In the dose escalation phase of the study, cohorts of new patients were treated with a single course of Resimmune as 15-minute infusions at doses ranging from 2.5 to 11.25µg/kg intravenously twice daily for 4 days. There was an expansion cohort at the maximal tolerated dose (MTD) in patients with stage IB-IIB CTCL and modified skin weighted assessment tool (mSWAT) scores of <50. In the expansion phase of the study, 13 patients received a single course at the 7.5µg/kg dose level. Patient eligibility-- Patients with CD3+ T cell malignancies diagnosed by morphologic, histochemical, and cell surface criteria and having failed a systemic therapy were eligible for the dose-escalation portion of the study. -

Acitretin; Adapalene; Alitretinoin; Bexarotene; Isotretinoin
8 February 2018 EMA/254364/2018 Pharmacovigilance Risk Assessment Committee (PRAC) Assessment report Referral under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data Retinoids containing medicinal products INN: Acitretin, Adapalene, Alitretinoin, Bexarotene, Isotretinoin, Tretinoin, Tazarotene Procedure number: EMEA/H/A-31/1446 Panretin EMEA/H/A-31/1446/C/000279/0037 Targretin EMEA/H/A-31/1446/C/000326/0043 Note: Assessment report as adopted by the PRAC and considered by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ......................................................................................... 2 1. Information on the procedure ................................................................. 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction......................................................................................................... 3 2.2. Clinical aspects .................................................................................................... 5 2.3. Data on efficacy .................................................................................................. -

Acitretin; Adapalene; Alitretinoin; Bexarotene; Isotretinoin
21 June 2018 EMA/261767/2018 Updated measures for pregnancy prevention during retinoid use Warning on possible risk of neuropsychiatric disorders also to be included for oral retinoids On 22 March 2018, the European Medicines Agency (EMA) completed its review of retinoid medicines, and confirmed that an update of measures for pregnancy prevention is needed. In addition, a warning on the possibility that neuropsychiatric disorders (such as depression, anxiety and mood changes) may occur will be included in the prescribing information for oral retinoids (those taken by mouth). Retinoids include the active substances acitretin, adapalene, alitretinoin, bexarotene, isotretinoin, tazarotene and tretinoin. They are taken by mouth or applied as creams or gels to treat several conditions mainly affecting the skin, including severe acne and psoriasis. Some retinoids are also used to treat certain forms of cancer. The review confirmed that oral retinoids can harm the unborn child and must not be used during pregnancy. In addition, the oral retinoids acitretin, alitretinoin and isotretinoin, which are used to treat conditions mainly affecting the skin, must be used in accordance with the conditions of a new pregnancy prevention programme by women able to have children. Topical retinoids (those applied to the skin) must also not be used during pregnancy, and by women planning to have a baby. More information is available below. Regarding the risk of neuropsychiatric disorders, the limitations of the available data did not allow to clearly establish whether this risk was due to the use of retinoids. However, considering that patients with severe skin conditions may be more vulnerable to neuropsychiatric disorders due to the nature of the disease, the prescribing information for oral retinoids will be updated to include a warning about this possible risk. -

Editorial Retinoids Induced Cancer Stem Cell Differentiation And
Editorial Retinoids Induced Cancer Stem Cell Differentiation and Apoptosis for Cancer Therapies Xin Cao Project Leader of Targeted Drug Design and Validation, Zhongshan Hospital Institute of Clinical Science, Fudan University Shanghai Medical College, Shanghai, 200032, China E-mail: [email protected] Received 31 January 2019; Accepted 03 April 2019; Publication 19 April 2019 Abstract Retinoids show great potential in various kinds of cancer chemotherapy due to its ability to induce signals for cell differentiation or death, as well as inhibit cancer stem cell proliferation. This paper summarized the recent progress of retinoids induced cancer stem cell differentiation and apoptosis in cancer therapy field, with the highlighted novel retinoid named WYC-209 in our lab, which could inhibit the tumor stem cell and malignant melanoma tumors with high efficacy and little toxicity. Keywords: Retinoids, cancer stem cell, differentiation, apoptosis. The discovery of retinoic acid receptors (RARs) from early research elucidates how vitamin A (Figure 1(A)) regulates mammalian physiology including spermatogenesis, fertilization, pregnancy maintenance, morphogenesis and organogenesis. Then studies indicate the main metabolized active form of VitaminA, all-trans retinoic acid (ATRA, Figure 1(B)), played important roles in certain DNAactivation and protein expression regulations [1].Accordingly, Molecular and Cellular Therapies, Vol. 7 1, 1–8. doi: 10.13052/mct2052-8426.711 This is an Open Access publication. c 2019 the Author(s). All rights reserved. 2 Xin Cao Figure 1 Structures of several retinoids. retinoids firstly occurred as naturally low-molecular weight, fat-soluble unsaturated isoprenoids, including retinaldehyde, 9-cis retinoic acid and 13-cis retinoic acid et al., which also played essential roles as all-trans retinoic acid (ATRA) in various aspects of human vision, immunity, as well as fetal cell proliferations [2]. -

Standard Oncology Criteria C16154-A
Prior Authorization Criteria Standard Oncology Criteria Policy Number: C16154-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE DUE BEFORE 03/2016 12/2/2020 1/26/2022 HCPCS CODING TYPE OF CRITERIA LAST P&T APPROVAL/VERSION N/A RxPA Q1 2021 20210127C16154-A PRODUCTS AFFECTED: See dosage forms DRUG CLASS: Antineoplastic ROUTE OF ADMINISTRATION: Variable per drug PLACE OF SERVICE: Retail Pharmacy, Specialty Pharmacy, Buy and Bill- please refer to specialty pharmacy list by drug AVAILABLE DOSAGE FORMS: Abraxane (paclitaxel protein-bound) Cabometyx (cabozantinib) Erwinaze (asparaginase) Actimmune (interferon gamma-1b) Calquence (acalbrutinib) Erwinia (chrysantemi) Adriamycin (doxorubicin) Campath (alemtuzumab) Ethyol (amifostine) Adrucil (fluorouracil) Camptosar (irinotecan) Etopophos (etoposide phosphate) Afinitor (everolimus) Caprelsa (vandetanib) Evomela (melphalan) Alecensa (alectinib) Casodex (bicalutamide) Fareston (toremifene) Alimta (pemetrexed disodium) Cerubidine (danorubicin) Farydak (panbinostat) Aliqopa (copanlisib) Clolar (clofarabine) Faslodex (fulvestrant) Alkeran (melphalan) Cometriq (cabozantinib) Femara (letrozole) Alunbrig (brigatinib) Copiktra (duvelisib) Firmagon (degarelix) Arimidex (anastrozole) Cosmegen (dactinomycin) Floxuridine Aromasin (exemestane) Cotellic (cobimetinib) Fludara (fludarbine) Arranon (nelarabine) Cyramza (ramucirumab) Folotyn (pralatrexate) Arzerra (ofatumumab) Cytosar-U (cytarabine) Fusilev (levoleucovorin) Asparlas (calaspargase pegol-mknl Cytoxan (cyclophosphamide) -

The Selective Retinoid X Receptor Agonist Bexarotene (LGD1069, Targretin) Prevents and Overcomes Multidrug Resistance in Advanced Breast Carcinoma
824 The selective retinoid X receptor agonist bexarotene (LGD1069, Targretin) prevents and overcomes multidrug resistance in advanced breast carcinoma Wan-Ching Yen and William W. Lamph women (1). The American Cancer Society estimates that f215,990 women in the United States will be found to have Department of Molecular Oncology, Ligand Pharmaceuticals, Inc., San Diego, California breast cancer and 40,110 women will die from the disease in 2004. Although chemotherapy provides the major therapeutic modality for treatment of advanced breast cancer, the 5-year survival for disseminated breast carci- Abstract noma is <20% due to relapse with drug-resistant disease. Acquired drug resistance represents a major challenge in Several mechanisms of drug resistance have been charac- the therapeutic management of breast cancer patients. We terized in breast cancer cell lines. The best understood reported previously that the retinoid X receptor–selective mechanism is P-glycoprotein (Pgp)–mediated drug resis- agonist bexarotene (LGD1069, Targretin) was efficacious tance (2). Pgp has been shown to contribute to resistance to in treating animal models of tamoxifen-resistant breast natural product–based chemotherapeutic agents, including cancer. The goal of this study was to evaluate the effect of taxanes, anthracyclines, Vinca alkaloids, podophyllotoxins, bexarotene on development of acquired drug resistance and and camptothecins (3). The relationship among Pgp its role in overcoming acquired drug resistance in advanced expression, Pgp function, and drug resistance in clinical breast cancer. Paclitaxel, doxorubicin, and cisplatin were samples of breast carcinoma was best illustrated by chosen as model compounds to determine the effect of Mechetner et al. (4). These investigators showed that the bexarotene on the development of acquired drug resis- level of Pgp expression in freshly resected breast tumors tance. -

Cancer Drug Costs for a Month of Treatment at Initial Food
Cancer drug costs for a month of treatment at initial Food and Drug Administration approval Year of FDA Monthly Cost Monthly cost (2013 Generic name Brand name(s) approval (actual $'s) $'s) Vinblastine Velban 1965 $78 $575 Thioguanine, 6-TG Thioguanine Tabloid 1966 $17 $122 Hydroxyurea Hydrea 1967 $14 $97 Cytarabine Cytosar-U, Tarabine PFS 1969 $13 $82 Procarbazine Matulane 1969 $2 $13 Testolactone Teslac 1969 $179 $1,136 Mitotane Lysodren 1970 $134 $801 Plicamycin Mithracin 1970 $50 $299 Mitomycin C Mutamycin 1974 $5 $22 Dacarbazine DTIC-Dome 1975 $29 $125 Lomustine CeeNU 1976 $10 $41 Carmustine BiCNU, BCNU 1977 $33 $127 Tamoxifen citrate Nolvadex 1977 $44 $167 Cisplatin Platinol 1978 $125 $445 Estramustine Emcyt 1981 $420 $1,074 Streptozocin Zanosar 1982 $61 $147 Etoposide, VP-16 Vepesid 1983 $181 $422 Interferon alfa 2a Roferon A 1986 $742 $1,573 Daunorubicin, Daunomycin Cerubidine 1987 $533 $1,090 Doxorubicin Adriamycin 1987 $521 $1,066 Mitoxantrone Novantrone 1987 $477 $976 Ifosfamide IFEX 1988 $1,667 $3,274 Flutamide Eulexin 1989 $213 $399 Altretamine Hexalen 1990 $341 $606 Idarubicin Idamycin 1990 $227 $404 Levamisole Ergamisol 1990 $105 $187 Carboplatin Paraplatin 1991 $860 $1,467 Fludarabine phosphate Fludara 1991 $662 $1,129 Pamidronate Aredia 1991 $507 $865 Pentostatin Nipent 1991 $1,767 $3,015 Aldesleukin Proleukin 1992 $13,503 $22,364 Melphalan Alkeran 1992 $35 $58 Cladribine Leustatin, 2-CdA 1993 $764 $1,229 Asparaginase Elspar 1994 $694 $1,088 Paclitaxel Taxol 1994 $2,614 $4,099 Pegaspargase Oncaspar 1994 $3,006 $4,713 -

Retinoid Treatment of Skin Diseases
Review Eur J Dermatol 2015; 25(5): 384-91 Lisa BECKENBACH1 Retinoid treatment of skin diseases Jens M. BARON2 Hans F. MERK2 Harald LOFFLER¨ 1 , Retinoids (vitamin A and its metabolites) are potent natural regula- Philipp M. AMANN1 2 tors of cellular activities, including cell growth and differentiation, and they mediate many essential regulatory functions, especially in the skin. 1 Department of Dermatology, SLK Hospital Heilbronn, Am Gesundbrunnen Biologically active retinoids exert their effects by binding to nuclear 20 -26, 74078 Heilbronn, Germany retinoic acid receptors and retinoid-X-receptors. The group of pharma- 2 Department of Dermatology and cologically used retinoids include naturally occurring and chemically Allergology, RWTH Aachen University Hospital, Pauwelsstrasse 30, 52074 Aachen, synthesised vitamin A derivatives. Due to their influence on keratinocyte Germany proliferation, epidermal differentiation and keratinisation, retinoids are commonly used in the field of dermatopharmacology. For safe admin- Reprints: P. M. Amann istration of retinoids, in-depth information about adverse effects and <[email protected]> comprehensive information of the patient are important. This article gives an overview on the effects, use, and side-effects of topical and systemic retinoids in dermatology. Key words: dermatopharmacology, retinoid therapy, retinoids, vitamin Article accepted on 05/2/2015 A derivatives itamin A (all-trans retinol) and its naturally occurring and synthetic derivatives, collectively Nonaromatic endogenous retinoids (1st generation) V referred to as retinoids, exert a wide variety of O profound effects during embryogenesis, reproduction, reg- OH OH ulation of inflammation, cell growth and cell differentiation. Their decisive role as critical gene regulators is primarily Vitamin A (all-trans retinol) Tretinoin (all-trans retinoic acid) mediated by their interaction with the nuclear retinoic acid receptors (RAR) and retinoid-X-receptors (RXR) [1]. -

NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2010
NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2010 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2010 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health This document is in the public domain and may be freely copied or reprinted. DISCLAIMER Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health (NIOSH). In addition, citations to Web sites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these Web sites. ORDERING INFORMATION To receive documents or other information about occupational safety and health topics, contact NIOSH at Telephone: 1–800–CDC–INFO (1–800–232–4636) TTY:1–888–232–6348 E-mail: [email protected] or visit the NIOSH Web site at www.cdc.gov/niosh For a monthly update on news at NIOSH, subscribe to NIOSH eNews by visiting www.cdc.gov/niosh/eNews. DHHS (NIOSH) Publication Number 2010−167 September 2010 Preamble: The National Institute for Occupational Safety and Health (NIOSH) Alert: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings was published in September 2004 (http://www.cdc.gov/niosh/docs/2004-165/). In Appendix A of the Alert, NIOSH identified a sample list of major hazardous drugs. The list was compiled from infor- mation provided by four institutions that have generated lists of hazardous drugs for their respec- tive facilities and by the Pharmaceutical Research and Manufacturers of America (PhRMA) from the American Hospital Formulary Service Drug Information (AHFS DI) monographs [ASHP/ AHFS DI 2003]. -
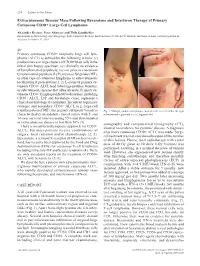
Extracutaneous Tumour Mass Following Bexarotene and Interferon Therapy of Primary Cutaneous CD30+ Large Cell Lymphoma
274 Letters to the Editor Extracutaneous Tumour Mass Following Bexarotene and Interferon Therapy of Primary Cutaneous CD30+ Large Cell Lymphoma Alexander Kreuter, Peter Altmeyer and Thilo Gambichler Department of Dermatology and Allergology, Ruhr-University Bochum, Gudrunstrasse 56, DE-44791 Bochum, Germany. E-mail: [email protected] Accepted November 11, 2005. Sir, Primary cutaneous CD30+ anaplastic large cell lym- phoma (ALCL) is defined by the following criteria: (i) predominance or large clusters of CD30+ blast cells in the initial skin biopsy specimen; (ii) clinically no evidence of lymphomatoid papulosis; (iii) no prior or concurrent lymphomatoid papulosis (LyP), mycosis fungoides (MF), or other type of cutaneous lymphoma or extracutaneous localization at presentation (1, 2). Lesions of primary cu- taneous CD30+ ALCL tend to be large nodules, tumours, or subcutaneous masses that often ulcerate. Primary cu- taneous CD30+ lymphoproliferative disorders, including CD30+ ALCL, LyP and borderline cases, represent a clinical and histological continuum. In contrast to primary systemic and secondary CD30+ ALCL (e.g. large-cell transformation of MF), the primary cutaneous variant is Fig. 1. Multiple nodules and plaques located in the (a) left axilla, (b) right characterized by an indolent clinical course with 5- and submammary region and (c) left inguinal fold. 10-year survival rates exceeding 95% and disseminated or extracutaneous disease in less than 10% (1). sonography and computerized tomography (CT), There is no uniform therapeutic approach for CD30+ showed no evidence for systemic disease. A diagnosis ALCL, but most patients receive combinations of of primary cutaneous CD30+ ACLC was made. Surgi- surgery, local radiation and/or chemotherapy (2, 3).