Low Ph Enhances the Glucosinolate-Mediated Yellowing of Takuan-Zuke Under Low Salt Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Keto & Vegan Menu
vegan sushi 100% plant-based options Vegetable Sushi Roll GFA 5 4pc 9 8pc Carrot, cucumber, avocado and cabbage roll with ginger and wasabi Avocado & Cucumber Uramaki GFA 7 6pc Inside out sushi roll with sesame, ginger and wasabi Spring Roll Uramaki 6 6pc With sweet chilli sauce Temaki Vegetable GFA 4.5 Avocado, cabbage, carrot, cucumber, red capsicum Hosomaki Kappa Maki GFA Small Sushi Roll 4.5 Cucumber sushi Hosomaki Avocado Maki GFA Small Sushi Roll 5 Avocado sushi Inari 5 2 pieces of sweet tofu pockets fill with sushi rice, no seaweed Teriyaki Tofu Balls GFA 6 4pc 8.5 6pc With avocado crush and chilli 11 8pc 13.5 10pc 16 12pc 18.5 14pc Nigiri Platter GFA 20 10 Pieces of mixed vegan nigiri, sushi rice topped with grilled pumpkin, shitake mushroom and inari tofu Yasai Itame Bento GFA 22 Vegan stir-fried vegetables, edamame, avocado uramaki and Japanese vegetable miso soup keto sushi Made to order using cauliflower ‘rice’ and cream cheese in place of regular rice Raw Salmon Roll GFA 7 4pc 11 8pc Fresh Ora King Salmon, avocado, cucumber 4pc cal 214 / carb 3.55g / fat 15.95g / prot 13.5g 8pc cal 428 / carb 7.1g / fat 31.9g / prot 27g Raw Tuna Roll GFA 7 4pc 11 8pc Fresh tuna, avocado and cucumber 4pc cal 159.5 / carb 3.55g / fat 9.8g / prot 13.35g 8pc cal 319 / carb 7.1g / fat 19.6g / prot 26.7g Veggie Roll GFA 5 4pc 9 8pc Red capsicum, cabbage, takuan (pickled daikon), avocado, cucumber and kewpie mayonnaise 4pc cal 115.5 / carb 5.5g / fat 9.3g / prot 2.15g 8pc cal 231 / carb 11g / fat 18.6g / prot 4.3g Grilled Chicken Roll GFA 6 4pc 10 -

The Hip Chick's Guide to Macrobiotics Audiobook Bonus: Recipes
1 The Hip Chick’s Guide to Macrobiotics Audiobook Bonus: Recipes and Other Delights Contents copyright 2009 Jessica Porter 2 DO A GOOD KITCHEN SET-UP There are some essentials you need in the kitchen in order to have macrobiotic cooking be easy and delicious. The essentials: • A sharp knife – eventually a heavy Japanese vegetable knife is best • Heavy pot with a heavy lid, enameled cast iron is best • Flame deflector or flame tamer, available at any cooking store • A stainless steel skillet • Wooden cutting board • Cast iron skillet Things you probably already have in your kitchen: • Blender • Strainer • Colander • Baking sheet • Slotted spoons • Wooden spoons • Mixing bowls • Steamer basket or bamboo steamer 3 You might as well chuck: • The microwave oven • Teflon and aluminum cookware Down the road: • Stainless-steel pressure cooker • Gas stove • Wooden rice paddles You know you’re really a macro when you own: • Sushi mats • Chopsticks • Suribachi with surikogi • Hand food mill • Juicer • Ohsawa pot • Nabe pot • Pickle press • A picture of George Ohsawa hanging in your kitchen! Stock your cupboard with: • A variety of grains • A variety of beans, dried • Canned organic beans • Dried sea vegetables • Whole wheat bread and pastry flour • A variety of noodles • Olive, corn, and sesame oil • Safflower oil for deep frying 4 • Shoyu, miso, sea salt • Umeboshi vinegar, brown rice vinegar • Mirin • Sweeteners: rice syrup, barley malt, maple syrup • Fresh tofu and dried tofu • Boxed silken tofu for sauces and creams • A variety of snacks from the health-food store • Tempeh • Whole wheat or spelt tortillas • Puffed rice • Crispy rice for Crispy Rice Treats • Organic apple juice • Amasake • Bottled carrot juice, but also carrots and a juicer • Agar agar • Kuzu • Frozen fruit for kanten in winter • Dried fruit • Roasted seeds and nuts • Raw vegetables to snack on • Homemade and good quality store- bought dips made of tofu, beans, etc. -
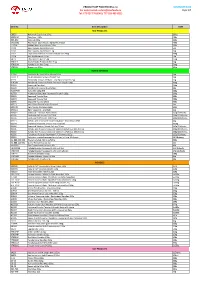
NOVEMBER 2020 Page
PRODUCT LIST TAKO FOODS s.r.o NOVEMBER 2020 For orders email: [email protected] Page 1/7 Tel: 773 027 776 (ENG), 777 926 495 (CZE) Item No. Item Description UoM RICE PRODUCTS 08930 Hakumaki Sushi rice 10 kg 10 kg 303013A Rice flour 400g 400g 839-840 Shiokoji 220g 220g CH16032 Rice Paper 22cm Round Spring-Roll 400g 400g J1371A Malted Rice - Kome Kouji 200g 200g J3100 Rice Toyama Koshihikari 5kg 5kg J3101 Rice Toyama Koshihikari 1 kg 1kg J3102 Toyama Koshihikari Funwari Gohan rice 200g 200g J3104 Rice Akitakomachi 5 kg 5kg J4075 Thai Jasmine Rice 25 kg 25kg YTK019 Koshi Yutaka Premium Rice 5 kg 5kg YTK023G Yutaka Sushi Rice 500g 500g YTK033 Brown rice 10kg 10kg NORI & SEAWEED 17164 Dashi Konbu Dried Kelp (Akaya) 1 kg 1kg CN-11-1 Chuka Wakame Seaweed salad 1kg 1kg K1131 Yamanaka Tokuyo Mehijiki - Dry Hijiki Seaweed 25g 25g K1450B Powdered Seaweed Aonori Premium Grade 100g 100g K1619 Seaweed Ogo Nori 500g K1628 Shredded Seaweed Kizami Nori 50g K1697WR Kaiso Mix 100g WR 100g K1703 Ariake Yaki Bara Nori Seaweed Grade A 100g 100g K2903 Seaweed Tosaka Blue 500g K2904 Seaweed Tosaka Red 500g K2905 Seaweed Tosaka White 500g K3214 Kelp Yama Dashi Konbu (Sokusei) 1kg K3217B Kelp Ma Konbu (Hakodate) 500g K3261A Hijiki Seawood - Me Hijiki 1kg K33021 Seaweed - Yakinori Aya Full Size 125g/50sheets K3305 Sushi nori full sheets 100/230G 230g/100sheets K3306 Sushi nori half sheets 100/125g 125g/100sheets K3313 Kofuku nori Seasoned seaweed Ajitsuke Nori Ohavo 8Pkt 24g K3314 Seasoned seaweed sesame nori chips 8G 30/8g K3340 Seaweed Yakinori Miyabi full -

Breakfast Bento 19 Anago Bento 30 Kamo-Kaki Meshi Bento 30
Okonomi / / Yuji Ramen TEL: 929.295.0480 :: 10:30AM - 7:00PM 7 DAYS A WEEK OKO BENTO MAZEMEN ALL SERVED WITH 7-GRAIN RICE (CONTAINS GLUTIN) (CONTAINS GLUTIN) ADD EXTRA NOODLES 1.50 Breakfast Bento 19 Bacon & Egg 18 roasted market fish, tsukemono, bacon, aji-tama egg aji tama, daily vegetables bonito flakes Anago Bento 30 Spicy Tuna 18 grilled sea-water eel, arima- tuna confit, sesame oil sansho, tsukemono, yuba fresh greens, togarashi *spicy Kamo-Kaki Meshi Bento 30 oysters braised in XO sauce (contains pork), Iro-Iro Midori 18 duck salad, garlic chips, arima sansho jalepeño-herb sauce, yuzu, white soy, fresh greens *vegan Hokkaido Bento 35 scallop & salmon sashimi, Maine uni, Uni Miso 23 red crab, ikura, tsukemono, shiso Maine uni, uni-miso butter, shiso OKO DONBURI SIDES // ADD-ONS ALL SERVED WITH 7-GRAIN RICE (CONTAINS GLUTIN) Aji Tama 2 Curry Rice 19 marinated soft boiled egg Japanese curry, roasted Miso Soup 3 market fish, takuan, fried garlic (contains glutin) Mabo Dofu 19 Add Bacon +4 shiitake, tofu, chili, Add Ikura +5 greens, pickles *spicy *vegan Add Maine Uni +8 Hawaiian Bowl 24 Orion 6 pulled pork, lomi salmon, Japanese beer tuna poke, inamona *no substitutions Follow us on Instagram for regular specials! @OKONOMIBK // @YUJIRAMEN_BK OKO PANTRY Retail items and common Japanese products for your home kitchen. DIY RAMEN KIT Shoyu Ramen Kit for 2 ......................................18 Miso Ramen Kit for 2 ........................................18 Veggie Miso Ramen Kit for 2 .............................18 JAPANESE PANTRY Original -

Catalogue 2017
Warabe Mura Wholefoods Hello, and welcome to Warabe Mura Wholefoods. We are a family owned mail order company situated in land locked Gifu Prefecture, central Japan. We are committed to offering what we consider to be the best and highest quality traditionally crafted whole foods available here. Foods that have been grown, whenever possible, organically and locally, processed as little as need be, with absolutely no artificial additives, colourings or flavourings used. Only natural occurring sweeteners such as brown rice and millet malts are used in the products we stock. All are sugar, dairy, egg, fish, fowl, and meat free. The natural personal care products are vegan. The house cleaners are environmentally friendly and all tissue & toilet papers are from recycled sources. We feel that to help heal the Earth and ourselves is enhanced by following a plant based diet, supporting sustainable organic agriculture, small farmers and producers of products that are Earth friendly and not manufactured at the expense of the environment, us or the creatures we share this world with. We specialise in Macrobiotic Whole Foods offering an extensive selection that includes shoyu, tamari, brown rice, umeboshi, miso as well as a wide variety of wild sea vegetables and Japanese pastas. We also offer a great range of imported organic whole foods such as dried fruit & nuts, pastas, sauces, fair trade teas & coffees, grains, flours and oils. Once a week we send out our seasonal organic vegetable box grown by dedicated organic farmers, and our delicious natural leavened breads, fresh out of the oven that day, are sent out twice a week. -

Ume Plum Vinegar Versus Apple Cider Vinegars
Ume plum vinegar versus apple cider vinegars Ume plum vinegar versus apple cider vinegars >>> Look Here <<< I was wondering if anyone has tried apple cider or umeboshi plum vinegar. I just had a lot of umeboshi plum vinegar tonight and hope I won't be paying for it.leaves). The red vinegar is fruity and very salty. Use over cooked or steamed invaluable ingredient in barbecue sauce, as the apple pairs seamlessly with rich, Vinegar cooking guide : Lappland 46 . - . , . , , h , , , , , , , . Veredus "" ! 2009 !. 35 / +1500 ,: 2013 vinegar Cooks use vinegar to make pickles, deglaze pans, marinate meats, and 10 Farmer- Favorite Simple Recipes, Straight From the Land 1- 2 tablespoons apple cider vinegar, ¼ cup brown rice vinegar 2 tablespoons ume plum vinegarplums, organic plum vinegar adds flavor to food without any calories. It's also We encourage you to shop, or simply browse and learn. Every Eden food has a story … where and how it is grown and crafted, its nutrition and health benefits autumn cider braises, where the vinegar Umeboshi Plum Vinegar.It's milder than wine or cider vinegars. apple cider vinegar. plum umeboshi vinegar = umeboshi plum vinegar = ume vinegar = ume plum vinegar = pickled or grains. This creates acetic acid, which gives the liquid a sour flavor. UnopenedDec 9, 2015 Apple Cider Vinegar. Source: Apples and/or hard apple cider. ACV is an A few people emailed me to ask about the sherry vinegar I used in yesterdays salad. If you can't find it, more rounded flavour than regular wine vinegars.A Japanese vinegar made with Umeboshi Plums, sea salt and shiso (parilla vinegar are different in flavor and ingredients. -

English Menu
‘Irasshaimase!’ Welcome to Abeno, Europe’s original specialist ‘Okonomi-yaki’ restaurant. We are delighted that you have chosen to sample one of Japan’s best-kept culinary secrets, a dish which has evolved over more than 400 years. We want you to be delighted with your experience today and, to that end, here are some tips towards attaining ‘Okonomi-yaki’ ‘あべの’ enlightenment. 心のこもった味づくり、心のこもったサービスに全力を注ぎます * Our menu is arranged in order of possible courses. Feel free, however, to order any dish, any time, in any order. 明るく暖かな空間を、態度と言葉の両方を使って演出します * The main courses - ‘okonomi-yaki ’, ‘yaki-soba’, ‘teppan-yaki’ etc - are sufficient for one. We would certainly recommend sharing dishes, so that you can enjoy a wider selection. If you are not sharing, we will do our best お客様一人一人、スタッフ一人一人を素直な気持ちで心から大切にします to get your chosen dishes to your table simultaneously. * All the ‘okonomi-yaki’ and ‘teppan-yaki’ are prepared in front of you. より質の高い仕事を目標に自分自身の能力を伸ばしてゆきます There is no need to do anything, unless you want to be involved. * All our ingredients are certified GMO-free and we source organic produce 食材や使用する物を通して地球環境を大切にすることを学び実践します wherever possible. Our meat is certified organic and additive-free and is from the Welsh Black Mountains. Our fish is sustainably-sourced. Our eggs are organic. We are members of the Sustainable Restaurant Association. ‘Abeno’ * We do have cutlery. * The ‘teppan’ (hot-plate) lives up to its name. Be very careful, particularly Every single guest is important to each of us. with children. Every single team member is important to each of us. * A discretionary charge for service of 12.5% will be added to your final bill. -

Tokyoya Ramen Menu
TOKYOYA RAMEN MENU APPETIZERS Gyoza (6) $6.99 Shrimp Shumai $7.95 6 Pieces. Steamed and pan fried pork and chicken Steamed or fried shrimp dumplings. potstickers. Edamame $5.95 Calamari (8) $9.25 Karaage (12) $8.95 Tender calamari legs, lightly battered and deep fried. Japanese style fried chicken. Fried Oysters (4) $9.95 Shrimp Katsu (3) $8.25 Panko breaded fried oysters. Panko breaded fried prawns. Chip & Dip $6.15 Potato Croquette $5.95 Fried gyoza skin chips with freshly pureed edamame dip. Deep fried panko breaded vegetable patties. Cha-Shu Steamed Bun (2) $6.95 Spicy Squid Balls $7.95 Seared pork belly and spring mix sandwiched in a fluffy Spicy. Deep fried squid balls coated with spices. bun. Takoyaki (8) $9.25 Bacon Wrapped Asparagus (9) $9.55 Fried balls of octopus in savory Japanese pancake batter. SALADS House Salad $6.75 Kimchee $4.95 Lettuce, tomato, carrots, corn, with a sesame dressing. Korean napa kimchee. RAMEN Tonkotsu Shio Ramen $12.25 Spicy Negi Ramen $12.75 Sea salt flavor. Sea salt soup base mild flavor. Pork bone Spicy. Soy sauce flavor. Spicy soy sauce soup base (rich broth seared cha-shu pork belly, bamboo, nori, tamago egg, flavor). Pork bone broth, rich flavor, seared cha-shu pork fish cake, scallion, black wood ear belly, bamboo, nori, sesame seeds, spicy teriyaki sauce and Tonkotsu Miso Ramen $13.25 a lot of scallions. Spicy. Most popular. Miso flavored rich flavor pork bone Tan Tan Ramen $13.75 broth, seared cha-shu pork belly, chili, bamboo, wakame, Spicy. -

Souvenirs to Help You Enjoy Vivid Memories of Your Trip to Atami
Famous Products – Local Specialties – Special Products Choose from a wealth of souvenirs to help you enjoy vivid memories of your trip to Atami. Atami Hot Springs, Japan’s leading hot spring village. Since long ago, hot springs have flowed from within the ocean, and because the ocean in the area was warm, the area was originally called “Atsuumigasaki” (literally, the “Peninsula of Hot Ocean Water”). It was said that soon after establishing the Edo shogunate, Tokugawa Ieyasu made a sojourn of seven days to bathe in the hot springs. After that, the Atami Hot Springs were “gifted” to Edo Castle as a famous hot spring village serving the Tokugawa family. So, though it was a hot spring resort town, Atami was “presented” as a “gift.” The delicious cuisine of the town… The “fruits of the earth”… The skills of the craftsmen… We present a catalog with a line up of every type of “souvenir” to enhance your memories of your trip to Atami. Reward your loved ones, and yourself, with some of these outstanding souvenirs. Foods / Side Dishes Side / Foods You can enjoy at home “Deep-fried” cooking condenses the Confectionary Western / Japanese simmered Kinmedai, a typical sh bounties from the sea giving birth to found in Izu,seasoned the way the company’s motto since its leading shermen chefs do. establishment: “We want customers to eat great tasting cuisine.” Foods / General Goods General / Foods Household GoodsHousehold Cafés / Restaurants / Cafés Services / Floor Maps Carrying on the traditions of five generations, the fifth generation owner Carrying on the sentiments of Suzuki Tomekichi, the founder who When it comes to “horse mackerel,” the fish is full of DHA and travels to the market every morning and buys only the seafood he is con- was the head of a group of fishermen and lived only for fishing, EPA which is considered to be good for the brain and blood, and vinced is suitable after examining it with his own eyes. -

Takuan (Pickled Daikon Radish)
Takuan (Pickled Daikon Radish) http://thedomesticman.com/2012/11/29/takuan-pickled-daikon-radish/ 2 large daikon radishes (approx 2 lbs) 3 cups water 1/4 cup sea salt 1/4 cup organic raw sugar 1/4 cup rice vinegar 1/4 tsp ground turmeric You may notice that this recipe calls for sugar, which is something I usually avoid in my recipes. Considering that most of the sugar is eaten by bacteria during the fermentation process, I’m not too worried about the sugar content of the final product. That being said, it still carries a little bit of a sweet taste, so it’s definitely not sugar-free. While modern Takuan is slightly sweet tasting, the original recipe likely didn’t have sugar in it, so feel free to omit the sugar if you’d like. Boil one cup of the water, then mix in the salt, sugar, and turmeric. Stir together until dissolved, then add the rest of the water and let it cool to room temperature. The turmeric may not completely dissolve, which is fine. As the water cools, peel your radishes and cut them into half-moons, about 1/4" thick. Fill up a half-gallon jar (or two quart-sized jars) with the sliced veggies. Once the water is cool, add the rice vinegar and pour everything into the jar. Add additional water if needed, until there’s about 1/2" of air at the top of the jar. Seal and leave in a dark part of your house for four or five days. -

Participating Restaurants in the Minokamo City Ouchi Gohan Programme
Participating Restaurants in the Minokamo City Ouchi Gohan Programme ●Prices include tax. ●Suggested items are available for takeout.●Please check with the restaurant as to whether you can eat in or not. Has a Pockettalk Translator Phone No. 1 Kamogawa Saryu Uokan 0574-25-0770 Address 505-0044 Kamogawa-cho 3-7-17 Opening Pick-up 11:00~18:00 Recommended Unagi Egg Bento (3 pcs.) ¥2,160 Hours Phone Orders 10:00~17:00 Item Yuki Hors D'Oeuvre Set (4~5 people) ¥8,640 Closed Thursdays Has a Pockettalk Translator Phone No. 2 Takuan Japanese Restaurant 0574-27-5788 Address 505-0035 Otemachi 2-81 Opening 11:00~14:00 Recommended Wagokoro Bento (Mondays: 2 Rice Dishes & 10 Sides ) ¥1,620 Hours 17:00~21:00 Item Fried Shrimp & Hire-katsu Bento ¥1,491 Closed -- Has a Pockettalk Translator Phone No. 3 Fukumi Ryokan Restaurant 0574-25-2850 Address 505-0041 Ota-cho 1896-3 Opening 11:00~22:00 Recommended Japanese Eel Bento ¥1,200 Hours Item Seasonal Bento ¥800 Closed -- Phone No. 4 Tom's So-ya 0574-25-3345 Address 505-0034 Kobi Shimokobi 2982-11 Opening 11:00~14:00 Recommended Demiglace Burger Patty Bento ¥1,134 Hours 17:00~22:00 Item Melty Omelette Rice Bento ¥864 Closed Mondays Has a Pockettalk Translator Phone No. 5 Suzume Tonkatsu Restaurant 0574-25-2918 Address 505-0041 Ota-cho 1868-2 Opening 11:30~19:30 Recommended Bono Pork Tonkatsu Bento ¥1,100 Hours Item Bono Pork Tonteki Bento ¥1,100 Closed Mondays & Tuesdays Phone No. -

Ohsawa® Nama® Shoyu
TMTM Fall/Winter 2015/2016 FREE SHIPPING* On All Orders Over $95 *See details on page 14 Ohsawa® The Most Trusted Name in Macrobiotics Sustainable Lifestyle Products... Organic • Macrobiotic • Vegan • Raw • Kosher • Gluten-Free Dear Friends, Welcome to Gold Mine Natural Food Company, your source for the best quality traditional, organic foods to support your health and happiness. Whether you’re new to healthy eating or a gourmet cook, we have what you’re looking for. In fact, using our quality ingredients will make your favorite dishes taste even better! It’s the time of year that many of us dream of relaxing in warm places or snuggling under a blanket by the fire. Some of my winter favorites include baking pumpkin or squash with our Ohsawa® Organic Genuine Mirin or using one of our fabulous misos in a quick and nourishing soup. Did you know you can generate more internal warmth and comfort by eating seasonally appropriate foods, and using longer cooking styles to create delicious, satisfying meals? What works for some people doesn’t always work for everybody. But our grandmothers were right when they told us, “eat your vegetables.” Perhaps your grandma didn’t include sea vegetables, but we do – every day! If you’re new to sea vegetables, start with something mild like organic nori, or garnish your soup with a dash of organic kelp granules. Low in calories and packed with minerals, your body will thank you for the added nourishment. And for a lively pick-me-up anytime, enjoy a mouthwatering shot of our new Organic Raw Kimchi Juice, Sauerkraut Juice, or Garlic Kraut Juice.