Native Pollinators: the Effects of Livestock Grazing on Montana
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historia Natural De Macrotera Pipiyolin (Hymenoptera: Andrenidae) En La Estación De Biología Chamela, Jalisco, México
Revista Mexicana de Biodiversidad 81: 87- 96, 2010 http://dx.doi.org/10.22201/ib.20078706e.2010.001.196 Historia natural de Macrotera pipiyolin (Hymenoptera: Andrenidae) en la Estación de Biología Chamela, Jalisco, México Natural history of Macrotera pipiyolin (Hymenoptera: Andrenidae) in the Estación de Biología Chamela, Jalisco, Mexico Beatriz Rodríguez-Velez1* y Ricardo Ayala2 1Instituto de Biología, Departamento de Zoología, Universidad Nacional Autónoma de México. Apartado postal 70-153, 04510 México, D.F., México. 2Estación de Biología Chamela, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 21, San Patricio, 48980 Jalisco, México. *Correspondencia: [email protected] Resumen. Se presenta información sobre la historia natural de Macrotera pipiyolin; el estudio se realizó en la Estación de Biología Chamela, Instituto de Biología, UNAM, entre junio y agosto de 1994. Las abejas emergieron poco después del inicio de la temporada de lluvias, su pico de actividad fue a principio de julio, presentando alrededor de 35 días activos. La actividad concuerda con la fl oración de Opuntia excelsa, planta de la que obtiene sus recursos. Las cópulas ocurren sobre las fl ores y los machos muestran 3 tipos de estrategias reproductivas: macho dominante, subordinado y patrullero. Las hembras son poliándricas y los machos polígamos. Las hembras construyen los nidos generalmente cerca de O. excelsa y tienen una distribución gregaria. Los nidos son simples, con un túnel principal y celdas, las que se cierran y desconectan del tunel una vez que se han aprovisionado y en ellas se ha depositado un huevo. Para la provisión de una celda se requiere de 2 a 18 cargas de polen. -
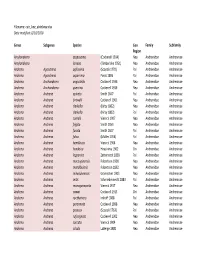
Anthophila List
Filename: cuic_bee_database. -

The Large Blue Butterfly Maculinea Alcon in Belgium: Science and Conservation
BULLETfN DE L'JNSTITUT ROYAL DES SCIENCES NATURELLES DE BELGIQUE BIOLOGIE, 72-S VPPL. : 183-1 85, 20Q ~ BULLETIN VAN HET KONINKLJJK BELGISCH INSTITVUT VOOR NATUURWETENSC HAPPEN BIOLOGLE, 72-S UPPL.: 183-1 85, 2002 The large blue butterfly Maculinea alcon in Belgium: science and conservation W. V ANREUSEL, H. VAN DYCK & D. MAES Introduction According to the Flemish Red List, M. alcon is threa tened. It is one of the few legally protected butterflies. In Belgium, the obligate ant-parasitic butterfly Maculinea Since 1996, both its complex life history and conserva alcon is confined to NE-Flanders (Kempen) and has tion biology have been studied rather intensively by our decreased considerably in distribution (fig. 1) and abun research team. Several populations went extinct, no less dance. than 9 did so in the 1990s including extinctions in nature reserves. Our analyses indicate that populations of smal- . ler, more isolated sites have a significantly higher extinc tion probability. At present, only 12 populations remain in 8 areas, including 5 nature reserves and 3 military areas (table I). Based on detailed egg counts, most populations show negative trends. We have recently finalised a Spe cies Action Plan for Maculinea a/con funded by the Flemish Ministry of Nature Conservation (VAN REUSEL et al. 2000). It was the first action plan for an invertebrate, but Flanders has only little experience with the imple mentation of such plans and with the integration of spe before 1991 cies-specific knowledge into site-oriented conservation. Hence, the M. a/con plan will be an important test-case. -

Components of Nest Provisioning Behavior in Solitary Bees (Hymenoptera: Apoidea)*
Apidologie 39 (2008) 30–45 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2007055 Review article Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea)* John L. Neff Central Texas Melittological Institute, 7307 Running Rope, Austin, Texas 78731, USA Received 13 June 2007 – Revised 28 September 2007 – Accepted 1 October 2007 Abstract – The components of nest provisioning behavior (resources per cell, transport capacity, trip dura- tion, trips per cell) are examined for a data set derived from the literature and various unpublished studies. While there is a trade-off between transport capacity and trips required per cell, the highest provisioning rates are achieved by bees carrying very large pollen loads at intermediate trip durations. Most solitary bees appear to be either egg or resource limited, so sustained provisioning rates over one cell per day are unusual. Provisioning rate / body size / transport capacity / solitary bees / trip duration 1. INTRODUCTION tradeoffs between provisioning one large cell or two small cells direct, indirect, or nonex- There is a vast literature on the foraging tac- istent? Are provisioning rates related to body tics of bees: what kinds of flowers to visit, how size? long to spend on a flower, how many flowers Provisioning rate is a general term and can in an inflorescence to visit, when to turn, and simply refer to the rate (mass per unit time) so forth. Much of this work is done within a at which foragers bring various food items framework of whether a forager should max- (pollen, nectar, oils, carrion, or whatever) to imize harvesting rate or foraging efficiency their nests. -

The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution
EN58CH04-Danforth ARI 5 December 2012 7:55 The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution Bryan N. Danforth,1∗ Sophie Cardinal,2 Christophe Praz,3 Eduardo A.B. Almeida,4 and Denis Michez5 1Department of Entomology, Cornell University, Ithaca, New York 14853; email: [email protected] 2Canadian National Collection of Insects, Agriculture Canada, Ottawa, Ontario K1A 0C6, Canada; email: [email protected] 3Institute of Biology, University of Neuchatel, Emile-Argand 11, 2009 Neuchatel, Switzerland; email: [email protected] 4Departamento de Biologia, FFCLRP-Universidade de Sao˜ Paulo, 14040-901 Ribeirao˜ Preto, Sao˜ Paulo, Brazil; email: [email protected] 5University of Mons, Laboratory of Zoology, 7000 Mons, Belgium; email: [email protected] Annu. Rev. Entomol. 2013. 58:57–78 Keywords First published online as a Review in Advance on Hymenoptera, Apoidea, bees, molecular systematics, sociality, parasitism, August 28, 2012 plant-insect interactions The Annual Review of Entomology is online at ento.annualreviews.org Abstract by 77.56.160.109 on 01/14/13. For personal use only. This article’s doi: Our understanding of bee phylogeny has improved over the past fifteen years 10.1146/annurev-ento-120811-153633 as a result of new data, primarily nucleotide sequence data, and new methods, Copyright c 2013 by Annual Reviews. primarily model-based methods of phylogeny reconstruction. Phylogenetic All rights reserved Annu. Rev. Entomol. 2013.58:57-78. Downloaded from www.annualreviews.org studies based on single or, more commonly, multilocus data sets have helped ∗ Corresponding author resolve the placement of bees within the superfamily Apoidea; the relation- ships among the seven families of bees; and the relationships among bee subfamilies, tribes, genera, and species. -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -

Do Not Eat Too Much! the Key to Success – Suitable Land Use Systems Research for the Scarce Large Blue
Part ne r o f N a t u r e NR.8 The key to success – Suitable land use systems The habitats of M. teleius need to be kept open by regular mowing or in a few cases by grazing. If mowing takes place too often or too infrequent it can lead to adverse habitat conditions for the host plant or the host ants. Adverse mowing can seriously reduce the chance of finding the right host ant and being adopted successfully. Another risk is mowing too early, which can lead to the destruction of the young caterpillars, which still live in the buds of the host plants. The wrong frequency or date of mowing can cause the local extinction of M. teleius populations. Research for the Scarce Large Blue The multitude of questions about how to design suit- The Scarce Large Blue (Maculinea teleius) has light grey- able land use systems in the different habitat types called brown underwings with two rows of dark spots. The for detailed research. The Bavarian Academy for Nature marginal spots often appear diffused. The butterfly is an Conservation and Landscape management (ANL) con- indicator species for extensively used moist mesophile sequently started a research project about the impact of grasslands like litter meadows, tall herb communities, and land uses systems on M. teleius. Fortunately we had the wet meadows, all mostly poor in nutrients. The species has chance to integrate our investigations in the EU research suffered a strong decrease all over Europe and is listed in project “MacMan” (EVK2-CT-2001-00126) which brought Appendix II and IV of the EU Habitats Directive. -

The Very Handy Bee Manual
The Very Handy Manual: How to Catch and Identify Bees and Manage a Collection A Collective and Ongoing Effort by Those Who Love to Study Bees in North America Last Revised: October, 2010 This manual is a compilation of the wisdom and experience of many individuals, some of whom are directly acknowledged here and others not. We thank all of you. The bulk of the text was compiled by Sam Droege at the USGS Native Bee Inventory and Monitoring Lab over several years from 2004-2008. We regularly update the manual with new information, so, if you have a new technique, some additional ideas for sections, corrections or additions, we would like to hear from you. Please email those to Sam Droege ([email protected]). You can also email Sam if you are interested in joining the group’s discussion group on bee monitoring and identification. Many thanks to Dave and Janice Green, Tracy Zarrillo, and Liz Sellers for their many hours of editing this manual. "They've got this steamroller going, and they won't stop until there's nobody fishing. What are they going to do then, save some bees?" - Mike Russo (Massachusetts fisherman who has fished cod for 18 years, on environmentalists)-Provided by Matthew Shepherd Contents Where to Find Bees ...................................................................................................................................... 2 Nets ............................................................................................................................................................. 2 Netting Technique ...................................................................................................................................... -

Adoption of Parasitic Maculinea Alcon Caterpillars (Lepidoptera: Lycaenidae) by Three Myrmica Ant Species
ANIMAL BEHAVIOUR, 2001, 62, 99–106 doi:10.1006/anbe.2001.1716, available online at http://www.idealibrary.com on Adoption of parasitic Maculinea alcon caterpillars (Lepidoptera: Lycaenidae) by three Myrmica ant species THOMAS DAMM ALS*, DAVID R. NASH*† & JACOBUS J. BOOMSMA*† *Department of Ecology and Genetics, University of Aarhus †Zoological Institute, Department of Population Ecology, University of Copenhagen, Denmark (Received 10 July 2000; initial acceptance 14 October 2000; final acceptance 31 January 2001; MS. number: 6631R) Maculinea butterflies are parasites of Myrmica ant nests. The Alcon blue, Maculinea alcon, is unusual in that it parasitizes the nests of several Myrmica species, using M. rubra, M. ruginodis and M. scabrinodis as hosts in different parts of Europe. In Denmark it uses M. rubra and M. ruginodis, but never M. scabrinodis. Some populations use one of these species exclusively, despite the presence of the alternative host, while others use both hosts simultaneously. To examine the basis of this specificity, and local coadaptation between host and parasite, we offered freshly emerged caterpillars of M. alcon from three populations differing in their host use to laboratory nests of all three recorded host ant species collected from each of the M. alcon populations. We measured the attractiveness of the caterpillars to their host ants as the time taken for them to be adopted by each ant colony. Caterpillars from all populations took longer to be adopted to M. scabrinodis nests than to nests of the other two ant species. Adoption times to M. rubra and M. ruginodis colonies differed: caterpillars from each of the two populations that used a single host species were adopted most quickly by that species when local ant colonies were used. -

Large Blue Priority Species Factsheet
t e e h s t c a f Large Blue Maculinea arion Conservation status Priority Species in UK Biodiversity Action Plan. Fully protected under Section 9 of the Wildlife and Countryside Act (1981). Bern Convention (Annexe IV) and 1995-9 Restoration EC Habitats and Species Directive (Annexe IV). sites 1970-82 °+ Pre 1970 This is the largest and rarest of our blue butterflies, distinguished by the unmistakable row of black spots on its upper forewing. The Large Blue has a remarkable life cycle that involves spending most of the year within the nests of red ants, where the larvae feed on ant grubs. The species has always been rare in Britain but declined rapidly during the twentieth century and became extinct in 1979. It has since been reintroduced successfully as part of a major conservation programme and by 2004 occurred on 9 sites. The Large Blue is declining throughout its world range and is a globally Endangered species. Life cycle The Large Blue is single brooded with adults flying from mid-June until late July. Eggs Foodplants are laid on the young flower buds of Wild Thyme. The larvae subsequently burrow into Larvae feed initially on the flower-heads of the flower head to feed on the flowers and developing seeds. Females lay eggs on Wild Thyme Thymus polytrichus but later feed plants growing in a range of vegetation heights, but survival is best in short turf where on ant grubs within the nests of the Myrmica the host ant M.sabuleti is most abundant. When the larvae are around 4 mm long they red ants. -

Melissa 6, January 1993
The Melittologist's Newsletter Ronald J. McGinley. Bryon N. Danforth. Maureen J. Mello Deportment of Entomology • Smithsonian Institution. NHB-105 • Washington. DC 20560 NUMBER-6 January, 1993 CONTENTS COLLECTING NEWS COLLECTING NEWS .:....:Repo=.:..:..rt=on~Th.:..:.=ird=-=-PC=A..::.;M:.:...E=xp=ed=it=io:..o..n-------=-1 Report on Third PCAM Expedition Update on NSF Mexican Bee Inventory 4 Robert W. Brooks ..;::.LC~;.,;;;....;;...:...:...;....;...;;;..o,__;_;.c..="-'-'-;.;..;....;;;~.....:.;..;.""""""_,;;...;....________,;. Snow Entomological Museum .:...P.:...roposo.a:...::..;:;..,:=..ed;::....;...P_:;C"'-AM~,;::S,;::u.;...;rvc...;;e.L.y-'-A"'"-rea.:o..=s'------------'-4 University of Kansas Lawrence, KS 66045 Collecting on Guana Island, British Virgin Islands & Puerto Rico 5 The third NSF funded PCAM (Programa Cooperativo so- RESEARCH NEWS bre Ia Apifauna Mexicana) expedition took place from March 23 to April3, 1992. The major goals of this trip were ...:..T.o..:he;::....;...P.:::a::.::ra::.::s;:;;it:..::ic;....;;B::...:e:..:e:....::L=.:e:.:.ia;:;L'{XJd:..::..::..::u;,;;:.s....:::s.:..:.in.:..o~gc::u.:.::/a:o.:n;,;;:.s_____~7 to do springtime collecting in the Chihuahuan Desert and Decline in Bombus terrestris Populations in Turkey 7 Coahuilan Inland Chaparral habitats of northern Mexico. We =:...:::=.:.::....:::..:...==.:.:..:::::..:::.::...:.=.:..:..:=~:....=..~==~..:.:.:.....:..=.:=L-.--=- also did some collecting in coniferous forest (pinyon-juni- NASA Sponsored Solitary Bee Research 8 per), mixed oak-pine forest, and riparian habitats in the Si- ;:...;N:..::.o=tes;.,;;;....;o;,;,n:....:Nc..:.e.::..;st;:.;,i;,:,;n_g....:::b""-y....:.M=-'-e.;;,agil,;;a.;_:;c=h:..:..:ili=d-=B;....;;e....:::e..::.s______.....:::.8 erra Madre Oriental. Hymenoptera Database System Update 9 Participants in this expedition were Ricardo Ayala (Insti- '-'M:.Liss.:..:..:..;;;in..:....:g..;:JB<:..;ee:..::.::.::;.:..,;Pa:::..rt=s=?=::...;:_"'-L..;=c.:.:...c::.....::....::=:..,_----__;:_9 tuto de Biologia, Chamela, Jalisco); John L. -

Version 2014-04-28 Attached Is the Dynamic1 List of Migratory Landbird
African-Eurasian Migratory Landbirds Action Plan Annex 3: Species Lists Version 2014-04-28 Attached is the dynamic1 list of migratory landbird species that occur within the African Eurasian region according to the following definition: 1. Migratory is defined as those species recorded within the IUCN Species Information Service (SIS) and BirdLife World Bird Database (WBDB) as ‘Full Migrant’, i.e. species which have a substantial (>50%) proportion of the global population which migrates: - with the addition of Great Bustard Otis tarda which is listed on CMS Appendix I and is probably erroneously recorded as an altitudinal migrant within SIS and the WBDB - with the omission of all single-country endemic migrants, in order to conform with the CMS definition of migratory which requires a species to ‘cross one or more national jurisdictional boundaries,’; in reality this has meant the removal of only one species, Madagascar Blue-pigeon Alectroenas madagascariensis. However, it should be noted that removing single-country endemics is not strictly analogous with omitting species that do not cross political borders. It is quite possible for a migratory species whose range extends across multiple countries to contain no populations that actually cross national boundaries as part of their regular migration. 2. African-Eurasian is defined as Africa, Europe (including all of the Russian Federation and excluding Greenland), the Middle East, Central Asia, Afghanistan, and the Indian sub-continent. 3. Landbird is defined as those species not recorded in SIS and the WBDB as being seabirds, raptors or waterbirds, except for the following waterbird species that are recorded as not utilising freshwater habitats: Geronticus eremita, Geronticus calvus, Burhinus oedicnemus, Cursorius cursor and Tryngites subruficollis.