Components of Nest Provisioning Behavior in Solitary Bees (Hymenoptera: Apoidea)*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollination of Two Species of Ferocactus: Interactions Between Cactus-Specialist Bees and Their Host Plants
Functional Blackwell Publishing, Ltd. Ecology 2005 Pollination of two species of Ferocactus: interactions 19, 727–734 between cactus-specialist bees and their host plants M. E. MCINTOSH Department of Ecology and Evolutionary Biology, 1041 E. Lowell Street; BioSciences West, Room 310, University of Arizona, Tucson, AZ 85721, USA Summary 1. Resolving the controversy over the prevalence of generalization in plant–pollinator interactions requires field studies characterizing the pollination effectiveness of all a plant’s floral visitors. Herein, the pollination effectiveness of all visitors to two species of barrel cactus (Ferocactus) was quantified. 2. Flowers of both species were pollinated almost exclusively by cactus-specialist bees: 99% (F. cylindraceus (Engelm.) Orcutt) and 94% (F. wislizeni (Engelm.) Britt. and Rose) of all seeds produced in this study resulted from cactus bee visits. 3. For F. cylindraceus, the cactus-specialist Diadasia rinconis was the most abundant visitor. For F. wislizeni, three cactus-specialists (including D. rinconis) plus generalists in the family Halictidae (which did not act as pollinators) each accounted for a quarter of all visits. 4. Diadasia rinconis visits to F. wislizeni flowers were more effective (per-visit) than visits by the other two cactus-specialists. 5. Pollen-collecting and nectar-collecting visits were equally effective. Nectar-collecting visits were the most abundant. 6. Apart from the non-pollinating halictids, floral visitors surprisingly did not include commonly co-occurring generalist bees. 7. These data suggest that, just as apparently specialized flowers may be visited by a diverse assemblage of generalists, so apparently generalized flowers may be visited predominantly by specialists, and that these specialists may perform virtually all of the pollination. -

Southwestern Rare and Endangered Plants
Preliminary Report on the Reproductive Biology of the Threatened Chisos Mountain Hedgehog Cactus BONNIE B. AMOS and CHRISTOS VASSILIOU Angelo State University, Texas Abstract: The Chisos Mountain hedgehog cactus (Echinocereus chisoensis, Cactaceae) is a narrow endemic restricted to an approximately 100 square mile area in Big Bend National Park, Texas. It was listed as threatened in 1987 as Echinocereus chisoensis var. chisoensis. An investigation of the reproductive biology and pollination ecology conducted in 1999 and 2000 revealed the taxon to be homogamous, self-incompatible, xenogamous, and heavily dependent upon the cactus oligolectic bee, Diadasia rinconis (Anthophoridae) for pollination. Despite infrequent bee visitation, fruit set from open pollination is high and fruits produce large numbers of seeds. Predation in 2002, probably from rodents as a result of severe drought conditions, was severe on plants, flower buds, and fruits. The Chisos Mountain hedgehog cactus, or Chisos jillo (Opuntia leptocaulis DC.), ocotillo (Fouquieria pitaya (Echinocereus chisoensis W. Marshall), is 1 of splendens K. Kunth), leatherstem (Jatropha dioica V. 20 threatened or endangered cacti listed by the de Cervantes), lechuguilla (Agave lechuguilla J. U.S. Fish and Wildlife Service for Region 2 (http: Torrey), and ceniza (Leucophyl1umf)zltescens (J. Ber- // ecos. fws.gov/ webpage/ webpage-lead.htrnl? landier) I. M. Johnston). An earlier study (Hender- lead_region=2&type=L&listings=l).In 1987 it was shott et al. 1992) did not show specific E. chisoen- added to the federal lists (53 FR 38453) of en- sis-nurse plant associations, but rather showed dangered and threatened wildlife and plants as associations as a consequence of soil conditions threatened because of its restricted distribution, that provide a hospitablL environment for a diver- low numbers, loss of viability in existing popula- sity of species or the exploitation by E. -

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -

On the Pima County Multi-Species Conservation Plan, Arizona
United States Department of the Interior Fish and ,Vildlife Service Arizona Ecological Services Office 2321 West Royal Palm Road, Suite 103 Phoenix, Arizona 85021-4951 Telephone: (602) 242-0210 Fax: (602) 242-2513 In reply refer to: AESO/SE 22410-2006-F-0459 April 13, 2016 Memorandum To: Regional Director, Fish and Wildlife Service, Albuquerque, New Mexico (ARD-ES) (Attn: Michelle Shaughnessy) Chief, Arizona Branch, Re.. gul 7/to . D'vision, Army Corps of Engineers, Phoenix, Arizona From: Acting Field Supervisor~ Subject: Biological and Conference Opinion on the Pima County Multi-Species Conservation Plan, Arizona This biological and conference opinion (BCO) responds to the Fish and Wildlife Service (FWS) requirement for intra-Service consultation on the proposed issuance of a section lO(a)(l)(B) incidental take permit (TE-84356A-O) to Pima County and Pima County Regional Flood Control District (both herein referenced as Pima County), pursuant to section 7 of the Endangered Species Act of 1973 (U.S.C. 1531-1544), as amended (ESA), authorizing the incidental take of 44 species (4 plants, 7 mammals, 8 birds, 5 fishes, 2 amphibians, 6 reptiles, and 12 invertebrates). Along with the permit application, Pima County submitted a draft Pima County Multi-Species Conservation Plan (MSCP). On June 10, 2015, the U.S. Army Corps of Engineers (ACOE) requested programmatic section 7 consultation for actions under section 404 of the Clean Water Act (CW A), including two Regional General Permits and 16 Nationwide Permits, that are also covered activities in the MSCP. This is an action under section 7 of the ESA that is separate from the section 10 permit issuance to Pima Couny. -

Insect Societies As Divided Organisms: the Complexities of Purpose and Cross-Purpose
Insect societies as divided organisms: The complexities of purpose and cross-purpose Joan E. Strassmann* and David C. Queller Department of Ecology and Evolutionary Biology, Rice University, Houston, TX 77005 Individual organisms are complex in a special way. The organiza- explained aspects of biology that were nonadaptive conse- tion and function of their parts seem directed toward a purpose: quences of history, from vestigial organs and other homologies the survival and reproduction of that individual. Groups of organ- to biogeographical patterns. Our understanding that organisms isms are different. They may also be complex, but that is usually are a mix of historical constraint and adaptation by natural because their parts, the individual organisms, are working at selection has led to many successful predictions about the natural cross-purposes. The most obvious exception to this rule is the social world, whereas Paley’s theory stands mute about the details. In insects. Here, the individuals cooperate in complex ways toward other words, Darwin’s theory is much richer than a simple the common goal of the success of the colony, even if it means that explanation for design; it makes many further extensions and most of them do not reproduce. Kin selection theory explains how predictions. Some of these extensions and predictions were not this can evolve. Nonreproductive individuals help in the reproduc- fully appreciated in Darwin’s time. The last several decades have tion of their kin, who share and transmit their genes. Such help is seen increased attention to a further important question about most favored when individuals can give more to their kin than they the apparent design of organisms. -

Provisioning Behavior and the Estimation of Investment Ratios in a Solitary Bee, Calh'opsis (Hypomacrotera) Persimilis (Cockerell) (Hymenoptera: Andrenidae) Bryan N
Behav Ecol Sociobiol (1990) 27:159-168 Behavioral Ecology and Sociobiology O Springer-Verlag 1990 Provisioning behavior and the estimation of investment ratios in a solitary bee, Calh'opsis (Hypomacrotera) persimilis (Cockerell) (Hymenoptera: Andrenidae) Bryan N. Danforth Snow Entomological Museum, Department of Entomology, University of Kansas, Lawrence, KS 66045-2106, USA Summary, One aspect of behavioral ecology that has male offspring production were calculated. Estimates of received considerable attention, especially by students the cost ratio based on the amount of time spent forag- of social insects, is the relative amount of energy invested ing, adult dry body weight, and pollen ball dry weight by parents in the rearing of male versus female offspring. all give similar values. Female offspring receive an ener- Sexual selection theory makes predictions about how getic investment of from 1.3 to 1.5 times that of males. individuals should allocate their total investment in the These results support the use of adult dry body weight sexes. To test these predictions we must accurately quan- ratios in the estimation of cost ratios. tify the relative "cost" incurred by a parent in the pro- duction of an average individual of either sex. Body weight ratios are the most common estimate of cost ra- tio, but the correspondence between offspring body Introduction weight and energetic investment on the part of the parent has rarely been determined. Calliopsis (Hypomaerotera) The relative amount of resources invested by parents persimilis is a solitary, ground-nesting bee whose natural in the total output of male versus female offspring is history makes it particularly convenient for studies of termed investment ratio. -

Historia Natural De Macrotera Pipiyolin (Hymenoptera: Andrenidae) En La Estación De Biología Chamela, Jalisco, México
Revista Mexicana de Biodiversidad 81: 87- 96, 2010 http://dx.doi.org/10.22201/ib.20078706e.2010.001.196 Historia natural de Macrotera pipiyolin (Hymenoptera: Andrenidae) en la Estación de Biología Chamela, Jalisco, México Natural history of Macrotera pipiyolin (Hymenoptera: Andrenidae) in the Estación de Biología Chamela, Jalisco, Mexico Beatriz Rodríguez-Velez1* y Ricardo Ayala2 1Instituto de Biología, Departamento de Zoología, Universidad Nacional Autónoma de México. Apartado postal 70-153, 04510 México, D.F., México. 2Estación de Biología Chamela, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 21, San Patricio, 48980 Jalisco, México. *Correspondencia: [email protected] Resumen. Se presenta información sobre la historia natural de Macrotera pipiyolin; el estudio se realizó en la Estación de Biología Chamela, Instituto de Biología, UNAM, entre junio y agosto de 1994. Las abejas emergieron poco después del inicio de la temporada de lluvias, su pico de actividad fue a principio de julio, presentando alrededor de 35 días activos. La actividad concuerda con la fl oración de Opuntia excelsa, planta de la que obtiene sus recursos. Las cópulas ocurren sobre las fl ores y los machos muestran 3 tipos de estrategias reproductivas: macho dominante, subordinado y patrullero. Las hembras son poliándricas y los machos polígamos. Las hembras construyen los nidos generalmente cerca de O. excelsa y tienen una distribución gregaria. Los nidos son simples, con un túnel principal y celdas, las que se cierran y desconectan del tunel una vez que se han aprovisionado y en ellas se ha depositado un huevo. Para la provisión de una celda se requiere de 2 a 18 cargas de polen. -
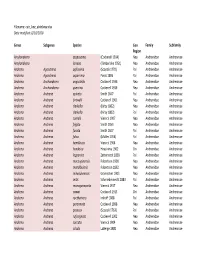
Anthophila List
Filename: cuic_bee_database. -

Comparative Methods Offer Powerful Insights Into Social Evolution in Bees Sarah Kocher, Robert Paxton
Comparative methods offer powerful insights into social evolution in bees Sarah Kocher, Robert Paxton To cite this version: Sarah Kocher, Robert Paxton. Comparative methods offer powerful insights into social evolution in bees. Apidologie, Springer Verlag, 2014, 45 (3), pp.289-305. 10.1007/s13592-014-0268-3. hal- 01234748 HAL Id: hal-01234748 https://hal.archives-ouvertes.fr/hal-01234748 Submitted on 27 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2014) 45:289–305 Review article * INRA, DIB and Springer-Verlag France, 2014 DOI: 10.1007/s13592-014-0268-3 Comparative methods offer powerful insights into social evolution in bees 1 2 Sarah D. KOCHER , Robert J. PAXTON 1Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA 2Institute for Biology, Martin-Luther-University Halle-Wittenberg, Halle, Germany Received 9 September 2013 – Revised 8 December 2013 – Accepted 2 January 2014 Abstract – Bees are excellent models for studying the evolution of sociality. While most species are solitary, many form social groups. The most complex form of social behavior, eusociality, has arisen independently four times within the bees. -

Novitates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM Novitates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 2640, pp. 1-24, figs. 1-36, tables 1-3 January 3, 1978 The Bionomics and Immature Stages of the Cleptoparasitic Bee Genus Protepeolus (Anthophoridae, Nomadinae) JEROME G. ROZEN, JR.,' KATHLEEN R. EICKWORT,2 AND GEORGE C. EICKWORT3 ABSTRACT Protepeolus singularis was found attacking cells numerous biological dissimilarities. The first in- in nests of Diadasia olivacea in southeastern Ari- star Protepeolus attacks and kills the pharate last zona. The following biological information is pre- larval instar of the host before consuming the sented: behavior of adult females while searching provisions, a unique feature for nomadine bees. for host nests; intraspecific interactions of fe- First and last larval instars and the pupa are males at the host nesting site; interactions with described taxonomically and illustrated. Brief host adults; oviposition; and such larval activities comparative descriptions of the other larval in- as crawling, killing the host, feeding, defecation, stars are also given. Larval features attest to the and cocoon spinning. In general, adult female be- common origin of Protepeolus and the other havior corresponds to that of other Nomadinae. Nomadinae. Cladistic analysis using 27 characters Females perch for extended periods near nest of mature larvae of the Nomadinae demonstrates entrances and avoid host females, which attack that Isepeolus is a sister group to all the other parasites when encountered. Females apparently Nomadinae known from larvae, including Pro- learn the locations of host nests and return to tepeolus, and that Protepeolus is a sister group to them frequently. -

2009 Pinon Canyon Invertebrate Survey Report
"- - 70.096 60.096 50.096 40.096 30.096 20.096 10.096 0.0% Fig. 1 Most abundant Apiformes species calculated as a proportion of the total abundance of Apiformes in the collection period. Pinon Canyon Maneuver Site, 2008. 04% 1 j 0.391> 0.2% 0.1% 0.0% Fig. 2 Least abundant Apiformes species calculated as a proportion of the total abundance of Apiformes in the collection period. Pinon Canyon Maneuver Site, 2008.7 Fig. 3 Most abundant Carabidae species calculated as a proportion of the total abundance of Carabidae in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 4 Least abundant Carabidae species calculated as a proportion of the total abundance of Carabidae in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 5 Asilidae species abundance calculated as a proportion of the total abundace of Asilidae in the collection period. Pinon Canyon Maneuver Site, 2008. 30.0% 25.0% 20.0% 15.0% 10.0% 5.0% 0.0% Fig. 6 Butterfly species abundance calculated as a proportion of the total abundance of butterflies in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 7 Most abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 8 Moderately abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 9 Least abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. -

The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution
EN58CH04-Danforth ARI 5 December 2012 7:55 The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution Bryan N. Danforth,1∗ Sophie Cardinal,2 Christophe Praz,3 Eduardo A.B. Almeida,4 and Denis Michez5 1Department of Entomology, Cornell University, Ithaca, New York 14853; email: [email protected] 2Canadian National Collection of Insects, Agriculture Canada, Ottawa, Ontario K1A 0C6, Canada; email: [email protected] 3Institute of Biology, University of Neuchatel, Emile-Argand 11, 2009 Neuchatel, Switzerland; email: [email protected] 4Departamento de Biologia, FFCLRP-Universidade de Sao˜ Paulo, 14040-901 Ribeirao˜ Preto, Sao˜ Paulo, Brazil; email: [email protected] 5University of Mons, Laboratory of Zoology, 7000 Mons, Belgium; email: [email protected] Annu. Rev. Entomol. 2013. 58:57–78 Keywords First published online as a Review in Advance on Hymenoptera, Apoidea, bees, molecular systematics, sociality, parasitism, August 28, 2012 plant-insect interactions The Annual Review of Entomology is online at ento.annualreviews.org Abstract by 77.56.160.109 on 01/14/13. For personal use only. This article’s doi: Our understanding of bee phylogeny has improved over the past fifteen years 10.1146/annurev-ento-120811-153633 as a result of new data, primarily nucleotide sequence data, and new methods, Copyright c 2013 by Annual Reviews. primarily model-based methods of phylogeny reconstruction. Phylogenetic All rights reserved Annu. Rev. Entomol. 2013.58:57-78. Downloaded from www.annualreviews.org studies based on single or, more commonly, multilocus data sets have helped ∗ Corresponding author resolve the placement of bees within the superfamily Apoidea; the relation- ships among the seven families of bees; and the relationships among bee subfamilies, tribes, genera, and species.