Southwestern Rare and Endangered Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DISTRIBUCIÓN Y ABUNDANCIA DE Jatropha Dioica EN EL CENTRO-NORTE DE MÉXICO
DISTRIBUCIÓN Y ABUNDANCIA DE Jatropha dioica EN EL CENTRO-NORTE DE MÉXICO DISTRIBUTION AND ABUNDANCE OF Jatropha dioica IN THE NORTHERN CENTER OF MEXICO Jesús M. Martínez-Calderas1, Jorge Palacio-Núñez1*, Juan F. Martínez-Montoya1, Genaro Olmos-Oropeza1 Fernando Clemente-Sánchez1, Gerardo Sánchez-Rojas2 1Colegio de Postgraduados, Campus San Luis Potosí, Postgrado de Innovación en Manejo de Recursos Naturales. Iturbide 73, Salinas de Hidalgo, San Luis Potosí. 78622, México. (bio- [email protected]), ([email protected]), ([email protected]), (olmosg@colpos. mx), ([email protected]). 2Universidad Autónoma del Estado de Hidalgo. Instituto de Ciencias Básicas e Ingeniería. Centro de Investigaciones Biológicas. Pachuca, Hidalgo, México. C.P. 42184. ([email protected]). RESUMEN ABSTRACT La planta sangre de grado (Jatropha dioica) habita en climas Leatherstem plants (Jatropha dioica) live in arid and áridos o semiáridos, se usa en medicina tradicional y podría semiarid climates, it is used in traditional medicine utilizarse como materia prima industrial, por lo cual tiene and could also be utilized as an industrial raw material, importancia económica para los pobladores rurales. Aspectos which is why this plant is vital to the economy of rural básicos sobre su distribución y abundancia se desconocen, populations. The fundamental aspects of its distribution así como los factores ambientales que las determinan. El and abundance are unknown, as well as the environmental objetivo de este estudio fue determinar su distribución, abun- factors that determine them. The objective of this study dancia y las variables que influyen en su densidad de tallos, was to determine its distribution, abundance, and the altura y biomasa de tallos. -

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -

Appendix F3 Rare Plant Survey Report
Appendix F3 Rare Plant Survey Report Draft CADIZ VALLEY WATER CONSERVATION, RECOVERY, AND STORAGE PROJECT Rare Plant Survey Report Prepared for May 2011 Santa Margarita Water District Draft CADIZ VALLEY WATER CONSERVATION, RECOVERY, AND STORAGE PROJECT Rare Plant Survey Report Prepared for May 2011 Santa Margarita Water District 626 Wilshire Boulevard Suite 1100 Los Angeles, CA 90017 213.599.4300 www.esassoc.com Oakland Olympia Petaluma Portland Sacramento San Diego San Francisco Seattle Tampa Woodland Hills D210324 TABLE OF CONTENTS Cadiz Valley Water Conservation, Recovery, and Storage Project: Rare Plant Survey Report Page Summary ............................................................................................................................... 1 Introduction ..........................................................................................................................2 Objective .......................................................................................................................... 2 Project Location and Description .....................................................................................2 Setting ................................................................................................................................... 5 Climate ............................................................................................................................. 5 Topography and Soils ......................................................................................................5 -

Cactus (Opuntia Spp.) As Forage 169
Cactus (Opuntia spp.) as forage 169 Food •••A.gricultv,.. Org•nU.taon or United -N••lon• FAO Cactus (Opuntiaspp.) PLANT PRODUCTION as forage AND PROTECTlON PAPER 169 Ed~ed by Candelario Mondragon-Jacobo lnstituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP) Mexico and Salvador Perez-Gonzalez Universidad Aut6noma de Queretaro Mexico Coordinated for FAD by Enrique Arias Horticultural Crops Group Stephen G. Reynolds Grassland and Pasture Crops Group FAO Plant Production and Protection Division and Manuel D. sanchez Feed Resources Group FAO Animal Production and HeaHh Division Produced within the frameworl< of the FAO International Technical Cooperation Networl< ot on Cactus Pear ••u nttttd• NaUon• Rome,2001 Reprinted 2002 The designations “developed” and “developing” economies are intended for statistical convenience and do not necessarily express a judgement about the stage reached by a particular country, country territory or area in the development process. The views expressed herein are those of the authors and do not necessarily represent those of the Food and Agriculture Organization of the United Nations or of their affiliated organization(s). The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-104705-7 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. -
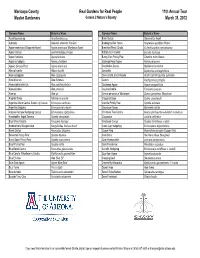
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Components of Nest Provisioning Behavior in Solitary Bees (Hymenoptera: Apoidea)*
Apidologie 39 (2008) 30–45 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2007055 Review article Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea)* John L. Neff Central Texas Melittological Institute, 7307 Running Rope, Austin, Texas 78731, USA Received 13 June 2007 – Revised 28 September 2007 – Accepted 1 October 2007 Abstract – The components of nest provisioning behavior (resources per cell, transport capacity, trip dura- tion, trips per cell) are examined for a data set derived from the literature and various unpublished studies. While there is a trade-off between transport capacity and trips required per cell, the highest provisioning rates are achieved by bees carrying very large pollen loads at intermediate trip durations. Most solitary bees appear to be either egg or resource limited, so sustained provisioning rates over one cell per day are unusual. Provisioning rate / body size / transport capacity / solitary bees / trip duration 1. INTRODUCTION tradeoffs between provisioning one large cell or two small cells direct, indirect, or nonex- There is a vast literature on the foraging tac- istent? Are provisioning rates related to body tics of bees: what kinds of flowers to visit, how size? long to spend on a flower, how many flowers Provisioning rate is a general term and can in an inflorescence to visit, when to turn, and simply refer to the rate (mass per unit time) so forth. Much of this work is done within a at which foragers bring various food items framework of whether a forager should max- (pollen, nectar, oils, carrion, or whatever) to imize harvesting rate or foraging efficiency their nests. -

Novitates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM Novitates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 2640, pp. 1-24, figs. 1-36, tables 1-3 January 3, 1978 The Bionomics and Immature Stages of the Cleptoparasitic Bee Genus Protepeolus (Anthophoridae, Nomadinae) JEROME G. ROZEN, JR.,' KATHLEEN R. EICKWORT,2 AND GEORGE C. EICKWORT3 ABSTRACT Protepeolus singularis was found attacking cells numerous biological dissimilarities. The first in- in nests of Diadasia olivacea in southeastern Ari- star Protepeolus attacks and kills the pharate last zona. The following biological information is pre- larval instar of the host before consuming the sented: behavior of adult females while searching provisions, a unique feature for nomadine bees. for host nests; intraspecific interactions of fe- First and last larval instars and the pupa are males at the host nesting site; interactions with described taxonomically and illustrated. Brief host adults; oviposition; and such larval activities comparative descriptions of the other larval in- as crawling, killing the host, feeding, defecation, stars are also given. Larval features attest to the and cocoon spinning. In general, adult female be- common origin of Protepeolus and the other havior corresponds to that of other Nomadinae. Nomadinae. Cladistic analysis using 27 characters Females perch for extended periods near nest of mature larvae of the Nomadinae demonstrates entrances and avoid host females, which attack that Isepeolus is a sister group to all the other parasites when encountered. Females apparently Nomadinae known from larvae, including Pro- learn the locations of host nests and return to tepeolus, and that Protepeolus is a sister group to them frequently. -

INDEX for 2011 HERBALPEDIA Abelmoschus Moschatus—Ambrette Seed Abies Alba—Fir, Silver Abies Balsamea—Fir, Balsam Abies
INDEX FOR 2011 HERBALPEDIA Acer palmatum—Maple, Japanese Acer pensylvanicum- Moosewood Acer rubrum—Maple, Red Abelmoschus moschatus—Ambrette seed Acer saccharinum—Maple, Silver Abies alba—Fir, Silver Acer spicatum—Maple, Mountain Abies balsamea—Fir, Balsam Acer tataricum—Maple, Tatarian Abies cephalonica—Fir, Greek Achillea ageratum—Yarrow, Sweet Abies fraseri—Fir, Fraser Achillea coarctata—Yarrow, Yellow Abies magnifica—Fir, California Red Achillea millefolium--Yarrow Abies mariana – Spruce, Black Achillea erba-rotta moschata—Yarrow, Musk Abies religiosa—Fir, Sacred Achillea moschata—Yarrow, Musk Abies sachalinensis—Fir, Japanese Achillea ptarmica - Sneezewort Abies spectabilis—Fir, Himalayan Achyranthes aspera—Devil’s Horsewhip Abronia fragrans – Sand Verbena Achyranthes bidentata-- Huai Niu Xi Abronia latifolia –Sand Verbena, Yellow Achyrocline satureoides--Macela Abrus precatorius--Jequirity Acinos alpinus – Calamint, Mountain Abutilon indicum----Mallow, Indian Acinos arvensis – Basil Thyme Abutilon trisulcatum- Mallow, Anglestem Aconitum carmichaeli—Monkshood, Azure Indian Aconitum delphinifolium—Monkshood, Acacia aneura--Mulga Larkspur Leaf Acacia arabica—Acacia Bark Aconitum falconeri—Aconite, Indian Acacia armata –Kangaroo Thorn Aconitum heterophyllum—Indian Atees Acacia catechu—Black Catechu Aconitum napellus—Aconite Acacia caven –Roman Cassie Aconitum uncinatum - Monkshood Acacia cornigera--Cockspur Aconitum vulparia - Wolfsbane Acacia dealbata--Mimosa Acorus americanus--Calamus Acacia decurrens—Acacia Bark Acorus calamus--Calamus -

The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution
EN58CH04-Danforth ARI 5 December 2012 7:55 The Impact of Molecular Data on Our Understanding of Bee Phylogeny and Evolution Bryan N. Danforth,1∗ Sophie Cardinal,2 Christophe Praz,3 Eduardo A.B. Almeida,4 and Denis Michez5 1Department of Entomology, Cornell University, Ithaca, New York 14853; email: [email protected] 2Canadian National Collection of Insects, Agriculture Canada, Ottawa, Ontario K1A 0C6, Canada; email: [email protected] 3Institute of Biology, University of Neuchatel, Emile-Argand 11, 2009 Neuchatel, Switzerland; email: [email protected] 4Departamento de Biologia, FFCLRP-Universidade de Sao˜ Paulo, 14040-901 Ribeirao˜ Preto, Sao˜ Paulo, Brazil; email: [email protected] 5University of Mons, Laboratory of Zoology, 7000 Mons, Belgium; email: [email protected] Annu. Rev. Entomol. 2013. 58:57–78 Keywords First published online as a Review in Advance on Hymenoptera, Apoidea, bees, molecular systematics, sociality, parasitism, August 28, 2012 plant-insect interactions The Annual Review of Entomology is online at ento.annualreviews.org Abstract by 77.56.160.109 on 01/14/13. For personal use only. This article’s doi: Our understanding of bee phylogeny has improved over the past fifteen years 10.1146/annurev-ento-120811-153633 as a result of new data, primarily nucleotide sequence data, and new methods, Copyright c 2013 by Annual Reviews. primarily model-based methods of phylogeny reconstruction. Phylogenetic All rights reserved Annu. Rev. Entomol. 2013.58:57-78. Downloaded from www.annualreviews.org studies based on single or, more commonly, multilocus data sets have helped ∗ Corresponding author resolve the placement of bees within the superfamily Apoidea; the relation- ships among the seven families of bees; and the relationships among bee subfamilies, tribes, genera, and species. -

Program 2014
CHRISTMAS MOUNTAINS RESEARCH SYMPOSIUM 2014 Terlingua Ranch Headquarters Brewster County, Texas May 19th–21st, 2014 Sponsored by the component institutions of the Texas State University System CHRISTMAS MOUNTAINS RESEARCH SYMPOSIUM Terlingua Ranch Headquarters Brewster County, Texas May 19th–21st, 2014 Monday, May 19th 6:00 pm Informal social for meeting participants by the swimming pool 7:00 pm Dinner available (on your own) at the Bad Rabbit Café Tuesday, May 20th 9:00 am Field trip to Christmas Mountains overlook (meet in the parking lot next to the swimming pool) 9:00 am Field trip to fluorspar mines (meet on the patio outside the café) 1:30 pm Paper session (in the bunkhouse beneath the Bad Rabbit Café) 8:00 pm Christmas Mountains Advisory Committee meeting (bunkhouse) Wednesday, May 21st 8:00 am Hike to Christmas Mountains overlook (meet in the parking lot next to the swimming pool) 8:30 am Field trip to Paisano Peak overlook (meet on the patio outside the café) 1:30 pm Paper session (in the bunkhouse beneath the Bad Rabbit Café) 7:00 pm Dinner for meeting registrants at the Bad Rabbit Café (regular dinner menu will be available for others at the café) - 2 - FIELD TRIPS Christmas Mountains Overlook Trip leader: Michael Huston Tuesday morning at 9:00 – meet in the parking lot next to the swimming pool Participants will carpool up the Old Mine Road to a scenic overlook at the road’s end. We will make several stops along the way to examine the geology and vegetation of the area and to take in views of the Rosillos Mountains and desert flats to the east and the Chisos Mountains to the southeast. -

List of Approved Plants
APPENDIX "X" – PLANT LISTS Appendix "X" Contains Three (3) Plant Lists: X.1. List of Approved Indigenous Plants Allowed in any Landscape Zone. X.2. List of Approved Non-Indigenous Plants Allowed ONLY in the Private Zone or Semi-Private Zone. X.3. List of Prohibited Plants Prohibited for any location on a residential Lot. X.1. LIST OF APPROVED INDIGENOUS PLANTS. Approved Indigenous Plants may be used in any of the Landscape Zones on a residential lot. ONLY approved indigenous plants may be used in the Native Zone and the Revegetation Zone for those landscape areas located beyond the perimeter footprint of the home and site walls. The density, ratios, and mix of any added indigenous plant material should approximate those found in the general area of the native undisturbed desert. Refer to Section 8.4 and 8.5 of the Design Guidelines for an explanation and illustration of the Native Zone and the Revegetation Zone. For clarity, Approved Indigenous Plants are considered those plant species that are specifically indigenous and native to Desert Mountain. While there may be several other plants that are native to the upper Sonoran Desert, this list is specific to indigenous and native plants within Desert Mountain. X.1.1. Indigenous Trees: COMMON NAME BOTANICAL NAME Blue Palo Verde Parkinsonia florida Crucifixion Thorn Canotia holacantha Desert Hackberry Celtis pallida Desert Willow / Desert Catalpa Chilopsis linearis Foothills Palo Verde Parkinsonia microphylla Net Leaf Hackberry Celtis reticulata One-Seed Juniper Juniperus monosperma Velvet Mesquite / Native Mesquite Prosopis velutina (juliflora) X.1.2. Indigenous Shrubs: COMMON NAME BOTANICAL NAME Anderson Thornbush Lycium andersonii Barberry Berberis haematocarpa Bear Grass Nolina microcarpa Brittle Bush Encelia farinosa Page X - 1 Approved - February 24, 2020 Appendix X Landscape Guidelines Bursage + Ambrosia deltoidea + Canyon Ragweed Ambrosia ambrosioides Catclaw Acacia / Wait-a-Minute Bush Acacia greggii / Senegalia greggii Catclaw Mimosa Mimosa aculeaticarpa var. -

Environmental Assessment Glass Mountain 480-Foot Guyed
Environmental Assessment Glass Mountain 480-foot Guyed Telecommunications Tower Approximately 27 Miles Northeast of Marathon, Brewster County, Texas June 2, 2010 Project No. 90107018 Prepared for: Permian Basin Regional Planning Commission Prepared by: Terracon Consultants, Inc. San Antonio, Texas June 2, 2010 Mr. Barney Welch Permian Basin Regional Planning Commission 2910 La Force Boulevard Midland, Texas 79706 Re: Draft Environmental Assessment Glass Mountain Tower Approximately 27 miles northeast of Marathon, Brewster County, Texas Prudent Project Number: 90107018 Dear Mr. Welch: Terracon has conducted a Draft Environmental Assessment and FCC NEPA Checklist of the proposed project with respect to the expected environmental impacts associated with grant funds issued by the Public Safety Interoperable Communications (PSIC) Grant Program, administered by the National Telecommunications and Information Administration (NTIA) of the U.S. Department of Commerce. The PSIC Grant Program is to assist State, local, tribal, and nongovernmental agencies in developing interoperable communications as they leverage newly available spectrum in the 700-800 megahertz (MHz) band. As a condition of the PSIC Grant Program, PSIC grantees must comply with all relevant Federal legislation. In addition, to the PSIC screening any new tower construction is required to undergo FCC NEPA Land Use screening in accordance with 47 CFR Section 1.1307 (a) (1) through (8), to determine whether any of the listed FCC special interest items would be significantly affected if a tower structure and/or antenna and associated equipment control cabinets were constructed at the proposed site location. The findings of this Draft Environmental and FCC NEPA Checklist are based on the project location, project type, and construction diagrams provided by the Pecos County Sheriff’s Department.