Carbon Sources of Benthic Fauna in Temperate Lakes Across Multiple Trophic States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Article: Inhibition of Methanogenic Archaea by Statins As a Targeted Management Strategy for Constipation and Related Disorders
Alimentary Pharmacology and Therapeutics Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders K. Gottlieb*, V. Wacher*, J. Sliman* & M. Pimentel† *Synthetic Biologics, Inc., Rockville, SUMMARY MD, USA. † Gastroenterology, Cedars-Sinai Background Medical Center, Los Angeles, CA, USA. Observational studies show a strong association between delayed intestinal transit and the production of methane. Experimental data suggest a direct inhibitory activity of methane on the colonic and ileal smooth muscle and Correspondence to: a possible role for methane as a gasotransmitter. Archaea are the only con- Dr K. Gottlieb, Synthetic Biologics, fi Inc., 9605 Medical Center Drive, rmed biological sources of methane in nature and Methanobrevibacter Rockville, MD 20850, USA. smithii is the predominant methanogen in the human intestine. E-mail: [email protected] Aim To review the biosynthesis and composition of archaeal cell membranes, Publication data archaeal methanogenesis and the mechanism of action of statins in this context. Submitted 8 September 2015 First decision 29 September 2015 Methods Resubmitted 7 October 2015 Narrative review of the literature. Resubmitted 20 October 2015 Accepted 20 October 2015 Results EV Pub Online 11 November 2015 Statins can inhibit archaeal cell membrane biosynthesis without affecting This uncommissioned review article was bacterial numbers as demonstrated in livestock and humans. This opens subject to full peer-review. the possibility of a therapeutic intervention that targets a specific aetiologi- cal factor of constipation while protecting the intestinal microbiome. While it is generally believed that statins inhibit methane production via their effect on cell membrane biosynthesis, mediated by inhibition of the HMG- CoA reductase, there is accumulating evidence for an alternative or addi- tional mechanism of action where statins inhibit methanogenesis directly. -

Phylogenetics of Archaeal Lipids Amy Kelly 9/27/2006 Outline
Phylogenetics of Archaeal Lipids Amy Kelly 9/27/2006 Outline • Phlogenetics of Archaea • Phlogenetics of archaeal lipids • Papers Phyla • Two? main phyla – Euryarchaeota • Methanogens • Extreme halophiles • Extreme thermophiles • Sulfate-reducing – Crenarchaeota • Extreme thermophiles – Korarchaeota? • Hyperthermophiles • indicated only by environmental DNA sequences – Nanoarchaeum? • N. equitans a fast evolving euryarchaeal lineage, not novel, early diverging archaeal phylum – Ancient archael group? • In deepest brances of Crenarchaea? Euryarchaea? Archaeal Lipids • Methanogens – Di- and tetra-ethers of glycerol and isoprenoid alcohols – Core mostly archaeol or caldarchaeol – Core sometimes sn-2- or Images removed due to sn-3-hydroxyarchaeol or copyright considerations. macrocyclic archaeol –PMI • Halophiles – Similar to methanogens – Exclusively synthesize bacterioruberin • Marine Crenarchaea Depositional Archaeal Lipids Biological Origin Environment Crocetane methanotrophs? methane seeps? methanogens, PMI (2,6,10,15,19-pentamethylicosane) methanotrophs hypersaline, anoxic Squalane hypersaline? C31-C40 head-to-head isoprenoids Smit & Mushegian • “Lost” enzymes of MVA pathway must exist – Phosphomevalonate kinase (PMK) – Diphosphomevalonate decarboxylase – Isopentenyl diphosphate isomerase (IPPI) Kaneda et al. 2001 Rohdich et al. 2001 Boucher et al. • Isoprenoid biosynthesis of archaea evolved through a combination of processes – Co-option of ancestral enzymes – Modification of enzymatic specificity – Orthologous and non-orthologous gene -

Dear Colleagues
NEW RECORDS OF CHIRONOMIDAE (DIPTERA) FROM CONTINENTAL FRANCE Joel Moubayed-Breil Applied ecology, 10 rue des Fenouils, 34070-Montpellier, France, Email: [email protected] Abstract Material recently collected in Continental France has allowed me to generate a list of 83 taxa of chironomids, including 37 new records to the fauna of France. According to published data on the chironomid fauna of France 718 chironomid species are hitherto known from the French territories. The nomenclature and taxonomy of the species listed are based on the last version of the Chironomidae data in Fauna Europaea, on recent revisions of genera and other recent publications relevant to taxonomy and nomenclature. Introduction French territories represent almost the largest Figure 1. Major biogeographic regions and subregions variety of aquatic ecosystems in Europe with of France respect to both physiographic and hydrographic aspects. According to literature on the chironomid fauna of France, some regions still are better Sites and methodology sampled then others, and the best sampled areas The identification of slide mounted specimens are: The northern and southern parts of the Alps was aided by recent taxonomic revisions and keys (regions 5a and 5b in figure 1); western, central to adults or pupal exuviae (Reiss and Säwedal and eastern parts of the Pyrenees (regions 6, 7, 8), 1981; Tuiskunen 1986; Serra-Tosio 1989; Sæther and South-Central France, including inland and 1990; Soponis 1990; Langton 1991; Sæther and coastal rivers (regions 9a and 9b). The remaining Wang 1995; Kyerematen and Sæther 2000; regions located in the North, the Middle and the Michiels and Spies 2002; Vårdal et al. -

Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China
Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China Di Wu Northeast Forestry University Caihong Zhao Northeast Forestry University Hui Bai Forestry Science Research Institute of Heilongjiang Province Fujuan Feng Northeast Forestry University Xin Sui Heilongjiang University Guangyu Sun ( [email protected] ) Northeast Forestry University Research article Keywords: Wetlands, Methanogens, Community diversity, Indicator species, Methanogenic metabolic patterns Posted Date: August 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-54821/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Background: Soil methanogenic microorganisms are one of the primary methane-producing microbes in wetlands. However, we still poorly understand the community characteristic and metabolic patterns of these microorganisms according to vegetation type and seasonal changes. Therefore, to better elucidate the effects of the vegetation type and seasonal factors on the methanogenic community structure and metabolic patterns, we detected the characteristics of the soil methanogenic mcrA gene from three types of natural wetlands in different seasons in the Xiaoxing'an Mountain region, China. Result: The results indicated that the distribution of Methanobacteriaceae (hydrogenotrophic methanogens) was higher in winter, while Methanosarcinaceae and Methanosaetaceae accounted for a higher proportion in summer. Hydrogenotrophic methanogenesis was the dominant trophic pattern in each wetland. The results of principal coordinate analysis and cluster analysis showed that the vegetation type considerably inuenced the methanogenic community composition. The methanogenic community structure in the Betula platyphylla – Larix gmelinii wetland was relatively different from the structure of the other two wetland types. -

1 U of Ill Urbana-Champaign PEET
U of Ill Urbana-Champaign PEET: A World Monograph of the Therevidae (Insecta: Diptera) Participant Individuals: CoPrincipal Investigator(s) : David K Yeates; Brian M Wiegmann Senior personnel(s) : Donald Webb; Gail E Kampmeier Post-doc(s) : Kevin C Holston Graduate student(s) : Martin Hauser Post-doc(s) : Mark A Metz Undergraduate student(s) : Amanda Buck; Melissa Calvillo Other -- specify(s) : Kristin Algmin Graduate student(s) : Hilary Hill Post-doc(s) : Shaun L Winterton Technician, programmer(s) : Brian Cassel Other -- specify(s) : Jeffrey Thorne Post-doc(s) : Christine Lambkin Other -- specify(s) : Ann C Rast Senior personnel(s) : Steve Gaimari Other -- specify(s) : Beryl Reid Technician, programmer(s) : Joanna Hamilton Undergraduate student(s) : Claire Montgomery; Heather Lanford High school student(s) : Kate Marlin Undergraduate student(s) : Dmitri Svistula Other -- specify(s) : Bradley Metz; Erica Leslie Technician, programmer(s) : Jacqueline Recsei; J. Marie Metz Other -- specify(s) : Malcolm Fyfe; David Ferguson; Jennifer Campbell; Scott Fernsler Undergraduate student(s) : Sarah Mathey; Rebekah Kunkel; Henry Patton; Emilia Schroer Technician, programmer(s) : Graham Teakle Undergraduate student(s) : David Carlisle; Klara Kim High school student(s) : Sara Sligar Undergraduate student(s) : Emmalyn Gennis Other -- specify(s) : Iris R Vargas; Nicholas P Henry Partner Organizations: Illinois Natural History Survey: Financial Support; Facilities; Collaborative Research Schlinger Foundation: Financial Support; In-kind Support; Collaborative Research 1 The Schlinger Foundation has been a strong and continuing partner of the therevid PEET project, providing funds for personnel (students, scientific illustrator, data loggers, curatorial assistant) and expeditions, including the purchase of supplies, to gather unknown and important taxa from targeted areas around the world. -

Checklist of the Family Chironomidae (Diptera) of Finland
A peer-reviewed open-access journal ZooKeys 441: 63–90 (2014)Checklist of the family Chironomidae (Diptera) of Finland 63 doi: 10.3897/zookeys.441.7461 CHECKLIST www.zookeys.org Launched to accelerate biodiversity research Checklist of the family Chironomidae (Diptera) of Finland Lauri Paasivirta1 1 Ruuhikoskenkatu 17 B 5, FI-24240 Salo, Finland Corresponding author: Lauri Paasivirta ([email protected]) Academic editor: J. Kahanpää | Received 10 March 2014 | Accepted 26 August 2014 | Published 19 September 2014 http://zoobank.org/F3343ED1-AE2C-43B4-9BA1-029B5EC32763 Citation: Paasivirta L (2014) Checklist of the family Chironomidae (Diptera) of Finland. In: Kahanpää J, Salmela J (Eds) Checklist of the Diptera of Finland. ZooKeys 441: 63–90. doi: 10.3897/zookeys.441.7461 Abstract A checklist of the family Chironomidae (Diptera) recorded from Finland is presented. Keywords Finland, Chironomidae, species list, biodiversity, faunistics Introduction There are supposedly at least 15 000 species of chironomid midges in the world (Armitage et al. 1995, but see Pape et al. 2011) making it the largest family among the aquatic insects. The European chironomid fauna consists of 1262 species (Sæther and Spies 2013). In Finland, 780 species can be found, of which 37 are still undescribed (Paasivirta 2012). The species checklist written by B. Lindeberg on 23.10.1979 (Hackman 1980) included 409 chironomid species. Twenty of those species have been removed from the checklist due to various reasons. The total number of species increased in the 1980s to 570, mainly due to the identification work by me and J. Tuiskunen (Bergman and Jansson 1983, Tuiskunen and Lindeberg 1986). -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -
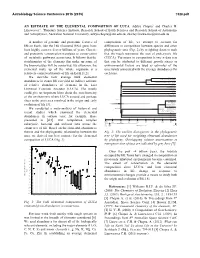
An Estimate of the Elemental Composition of Luca
Astrobiology Science Conference 2015 (2015) 7328.pdf AN ESTIMATE OF THE ELEMENTAL COMPOSITION OF LUCA. Aditya Chopra1 and Charles H. Lineweaver1, 1Planetary Science Institute, Research School of Earth Sciences and Research School of Astronomy and Astrophysics, Australian National University, [email protected], [email protected] A number of genomic and proteomic features of composition of life, we attempt to account for life on Earth, like the 16S ribosomal RNA gene, have differences in composition between species and other been highly conserved over billions of years. Genetic phylogenetic taxa (Fig. 2) by weighting datasets such and proteomic conservation translates to conservation that the result represents the root of prokaryotic life of metabolic pathways across taxa. It follows that the (LUCA). Variations in composition between data sets stoichiometry of the elements that make up some of that can be attributed to different growth stages or the biomolecules will be conserved. By extension, the environmental factors are used as estimates of the elemental make up of the whole organism is a uncertainty associated with the average abundances for relatively conserved feature of life on Earth [1,2]. each taxa. We describe how average bulk elemental Euryarchaeota 1a Methanococci, Methanobacteria, Methanopyri 7 Euryarchaeota 1b abundances in extant life can yield an indirect estimate 6 Thermoplasmata, Methanomicrobia, Halobacteria, Archaeoglobi Euryarchaeota 2 4 Thermococci of relative abundances of elements in the Last Crenarchaeota Sulfolobus, Thermoproteus ? Thaumarchaeota Universal Common Ancestor (LUCA). The results Cenarchaeum ? Korarchaeota ARMAN could give us important hints about the stoichiometry ? 2 Archaeal Richmond Mine Acidophilic Nanoorganisms Nanoarchaeota of the environment where LUCA existed and perhaps Archaea Terrabacteria clues to the processes involved in the origin and early Actinobacteria, Deinococcus-Thermus, 1 Cyanobacteria, Life Chloroflexi, 9 evolution of life [3]. -

Establishment and Analysis of Microbial Communities Capable of Producing Methane from Grass Waste at Extremely High C/N Ratio
International Journal of New Technology and Research (IJNTR) ISSN:2454-4116, Volume-2, Issue-9, September 2016 Pages 81-86 Establishment and Analysis of Microbial Communities Capable of Producing Methane from Grass Waste at Extremely High C/N Ratio S. Matsuda, T. Ohtsuki* Anaerobic digestion is a conventional method for biomass Abstract— Acclimation of microbial communities, aiming to utilization [8]. Despite the fact that a great deal of research methane production from grass as a sole substrate at extremely for methane production from grass has been conducted high carbon-to-nitrogen (C/N) ratio, was conducted. In a series especially in recent years, almost processes need supply of of experiments with various sizes of added grass, two microbial communities showing high methane production were obtained abundant sludge source such as sewage and manure [9, 10]. with powdered grass. In the two microbial communities Many studies indicated that the optimal carbon-to-nitrogen designated NR and RP, Bacteroidia including genera (C/N) ratio in methane fermentation were 25-30, and the C/N Bacteroides, Dysgonomonas, Proteiniphilum, and Alistipes were ratio higher than 40 are not generally suitable [11]. The C/N detected as dominant members in eubacteria. It was also shown ration of raw grass shows a wide range; wild grassy weed is at that Methanomicrobia and Methanobacteria including genera relatively high (>59) while lawn grass is at low (<29) [12]. Methanomassiliicoccus and Methanobacterium were found as dominant members in methanogen. It is noteworthy that Therefore, it is difficult to conduct methane fermentation nitrogen fixation were observed both in NR and RP, suggesting with wild grass-only, also being due to less degradability of that insufficiency of nitrogen sources would be complemented lignocellulose [13]. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

1 Development of Prediction Models of Methane Production by Sheep
Development of Prediction Models of Methane Production by Sheep and Cows Using Rumen Microbiota Data Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Boyang Zhang Graduate Program in Animal Sciences The Ohio State University 2018 Master's Examination Committee: Dr. Zhongtang Yu, Advisor Dr. Moraes, Co-advisor Dr. Firkins 1 Copyrighted by Boyang Zhang 2018 2 Abstract Methane emission from the rumen leads to approximately 10 % loss of the ingested energy, at the conversion of digestible to metabolizable energy. Besides research on mitigation of methane emission, much research interests have also been gravitated towards development of prediction models of methane emissions from livestock because the global warming is reducing agriculture productivity (Johnson and Johnson, 1995). An accurate prediction of enteric methane production from cattle and sheep can assist in balancing the increased livestock production with subsequent environmental impacts. Methane is an inevitable byproduct of the microbial fermentation processes in the rumen, and certain ruminal microbes have direct impacts to methane production (Morgavi et al., 2010). Thus, we hypothesized that the inclusion of individual microbial groups as predictor variables could improve the robustness and accuracy of prediction models. However, inclusion of microbial variables into prediction models can result in overfitting. Machine-learning algorithms can automatically select the key explanatory predictors, and Linear Mixed Models can provide a framework to predict random effects describing between animal variations. We proposed three novel frameworks for subset selections of microbial variables (MV) and one framework for generalized linear mixed models (GLMM) using L1-penalization (GLMMLASSO) selection with cross-validations (CV) ii to address the overfitting problems and to develop parsimonious prediction models of methane production. -

Lifestyle Preferences Drive the Structure and Diversity of Bacterial and Archaeal Communities in a Small Riverine Reservoir
www.nature.com/scientificreports OPEN Lifestyle preferences drive the structure and diversity of bacterial and archaeal communities in a small riverine reservoir Carles Borrego1,2, Sergi Sabater1,3* & Lorenzo Proia1,4 Spatial heterogeneity along river networks is interrupted by dams, afecting the transport, processing, and storage of organic matter, as well as the distribution of biota. We here investigated the structure of planktonic (free-living, FL), particle-attached (PA) and sediment-associated (SD) bacterial and archaeal communities within a small reservoir. We combined targeted-amplicon sequencing of bacterial and archaeal 16S rRNA genes in the DNA and RNA community fractions from FL, PA and SD, followed by imputed functional metagenomics, in order to unveil diferences in their potential metabolic capabilities within the reservoir (tail, mid, and dam sections) and lifestyles (FL, PA, SD). Both bacterial and archaeal communities were structured according to their life-style preferences rather than to their location in the reservoir. Bacterial communities were richer and more diverse when attached to particles or inhabiting the sediment, while Archaea showed an opposing trend. Diferences between PA and FL bacterial communities were consistent at functional level, the PA community showing higher potential capacity to degrade complex carbohydrates, aromatic compounds, and proteinaceous materials. Our results stressed that particle-attached prokaryotes were phylogenetically and metabolically distinct from their free-living counterparts, and that performed as hotspots for organic matter processing within the small reservoir. Spatial heterogeneity of river networks results from the sequence of lotic segments—which promote the transport and quick transformation of materials—and lentic segments such as large pools and wetlands—which mostly contribute to the process and storage of organic matter 1,2.