Review Article: Inhibition of Methanogenic Archaea by Statins As a Targeted Management Strategy for Constipation and Related Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methanobrevibacter Cuticularis Sp
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Oct. 1996, p. 3620–3631 Vol. 62, No. 10 0099-2240/96/$04.0010 Copyright q 1996, American Society for Microbiology Physiological Ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., Isolated from the Hindgut of the Termite Reticulitermes flavipes JARED R. LEADBETTER AND JOHN A. BREZNAK* Department of Microbiology and Center for Microbial Ecology, Michigan State University, East Lansing, Michigan 48824-1101 Received 11 January 1996/Accepted 15 July 1996 Two morphologically distinct, H2- and CO2-utilizing methanogens were isolated from gut homogenates of the subterranean termite, Reticulitermes flavipes (Kollar) (Rhinotermitidae). Strain RFM-1 was a short straight rod (0.4 by 1.2 mm), whereas strain RFM-2 was a slightly curved rod (0.34 by 1.6 mm) that possessed polar fibers. Their morphology, gram-positive staining reaction, resistance to cell lysis by chemical agents, and narrow range of utilizable substrates were typical of species belonging to the family Methanobacteriaceae. Analysis of the nearly complete sequences of the small-subunit rRNA-encoding genes confirmed this affiliation and supported their recognition as new species of Methanobrevibacter: M. cuticularis (RFM-1) and M. curvatus (RFM-2). The per cell rates of methanogenesis by strains RFM-1 and RFM-2 in vitro, taken together with their in situ population densities (ca. 106 cells z gut21; equivalent to 109 cells z ml of gut fluid21), could fully account for the rate of methane emission by the live termites. UV epifluorescence and electron microscopy confirmed that RFM-1- and RFM-2-type cells were the dominant methanogens in R. -

Phylogenetics of Archaeal Lipids Amy Kelly 9/27/2006 Outline
Phylogenetics of Archaeal Lipids Amy Kelly 9/27/2006 Outline • Phlogenetics of Archaea • Phlogenetics of archaeal lipids • Papers Phyla • Two? main phyla – Euryarchaeota • Methanogens • Extreme halophiles • Extreme thermophiles • Sulfate-reducing – Crenarchaeota • Extreme thermophiles – Korarchaeota? • Hyperthermophiles • indicated only by environmental DNA sequences – Nanoarchaeum? • N. equitans a fast evolving euryarchaeal lineage, not novel, early diverging archaeal phylum – Ancient archael group? • In deepest brances of Crenarchaea? Euryarchaea? Archaeal Lipids • Methanogens – Di- and tetra-ethers of glycerol and isoprenoid alcohols – Core mostly archaeol or caldarchaeol – Core sometimes sn-2- or Images removed due to sn-3-hydroxyarchaeol or copyright considerations. macrocyclic archaeol –PMI • Halophiles – Similar to methanogens – Exclusively synthesize bacterioruberin • Marine Crenarchaea Depositional Archaeal Lipids Biological Origin Environment Crocetane methanotrophs? methane seeps? methanogens, PMI (2,6,10,15,19-pentamethylicosane) methanotrophs hypersaline, anoxic Squalane hypersaline? C31-C40 head-to-head isoprenoids Smit & Mushegian • “Lost” enzymes of MVA pathway must exist – Phosphomevalonate kinase (PMK) – Diphosphomevalonate decarboxylase – Isopentenyl diphosphate isomerase (IPPI) Kaneda et al. 2001 Rohdich et al. 2001 Boucher et al. • Isoprenoid biosynthesis of archaea evolved through a combination of processes – Co-option of ancestral enzymes – Modification of enzymatic specificity – Orthologous and non-orthologous gene -

Supporting Information
Supporting Information Lozupone et al. 10.1073/pnas.0807339105 SI Methods nococcus, and Eubacterium grouped with members of other Determining the Environmental Distribution of Sequenced Genomes. named genera with high bootstrap support (Fig. 1A). One To obtain information on the lifestyle of the isolate and its reported member of the Bacteroidetes (Bacteroides capillosus) source, we looked at descriptive information from NCBI grouped firmly within the Firmicutes. This taxonomic error was (www.ncbi.nlm.nih.gov/genomes/lproks.cgi) and other related not surprising because gut isolates have often been classified as publications. We also determined which 16S rRNA-based envi- Bacteroides based on an obligate anaerobe, Gram-negative, ronmental surveys of microbial assemblages deposited near- nonsporulating phenotype alone (6, 7). A more recent 16S identical sequences in GenBank. We first downloaded the gbenv rRNA-based analysis of the genus Clostridium defined phylo- files from the NCBI ftp site on December 31, 2007, and used genetically related clusters (4, 5), and these designations were them to create a BLAST database. These files contain GenBank supported in our phylogenetic analysis of the Clostridium species in the HGMI pipeline. We thus designated these Clostridium records for the ENV database, a component of the nonredun- species, along with the species from other named genera that dant nucleotide database (nt) where 16S rRNA environmental cluster with them in bootstrap supported nodes, as being within survey data are deposited. GenBank records for hits with Ͼ98% these clusters. sequence identity over 400 bp to the 16S rRNA sequence of each of the 67 genomes were parsed to get a list of study titles Annotation of GTs and GHs. -

Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China
Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China Di Wu Northeast Forestry University Caihong Zhao Northeast Forestry University Hui Bai Forestry Science Research Institute of Heilongjiang Province Fujuan Feng Northeast Forestry University Xin Sui Heilongjiang University Guangyu Sun ( [email protected] ) Northeast Forestry University Research article Keywords: Wetlands, Methanogens, Community diversity, Indicator species, Methanogenic metabolic patterns Posted Date: August 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-54821/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Background: Soil methanogenic microorganisms are one of the primary methane-producing microbes in wetlands. However, we still poorly understand the community characteristic and metabolic patterns of these microorganisms according to vegetation type and seasonal changes. Therefore, to better elucidate the effects of the vegetation type and seasonal factors on the methanogenic community structure and metabolic patterns, we detected the characteristics of the soil methanogenic mcrA gene from three types of natural wetlands in different seasons in the Xiaoxing'an Mountain region, China. Result: The results indicated that the distribution of Methanobacteriaceae (hydrogenotrophic methanogens) was higher in winter, while Methanosarcinaceae and Methanosaetaceae accounted for a higher proportion in summer. Hydrogenotrophic methanogenesis was the dominant trophic pattern in each wetland. The results of principal coordinate analysis and cluster analysis showed that the vegetation type considerably inuenced the methanogenic community composition. The methanogenic community structure in the Betula platyphylla – Larix gmelinii wetland was relatively different from the structure of the other two wetland types. -

Development of the Equine Hindgut Microbiome in Semi-Feral and Domestic Conventionally-Managed Foals Meredith K
Tavenner et al. Animal Microbiome (2020) 2:43 Animal Microbiome https://doi.org/10.1186/s42523-020-00060-6 RESEARCH ARTICLE Open Access Development of the equine hindgut microbiome in semi-feral and domestic conventionally-managed foals Meredith K. Tavenner1, Sue M. McDonnell2 and Amy S. Biddle1* Abstract Background: Early development of the gut microbiome is an essential part of neonate health in animals. It is unclear whether the acquisition of gut microbes is different between domesticated animals and their wild counterparts. In this study, fecal samples from ten domestic conventionally managed (DCM) Standardbred and ten semi-feral managed (SFM) Shetland-type pony foals and dams were compared using 16S rRNA sequencing to identify differences in the development of the foal hindgut microbiome related to time and management. Results: Gut microbiome diversity of dams was lower than foals overall and within groups, and foals from both groups at Week 1 had less diverse gut microbiomes than subsequent weeks. The core microbiomes of SFM dams and foals had more taxa overall, and greater numbers of taxa within species groups when compared to DCM dams and foals. The gut microbiomes of SFM foals demonstrated enhanced diversity of key groups: Verrucomicrobia (RFP12), Ruminococcaceae, Fusobacterium spp., and Bacteroides spp., based on age and management. Lactic acid bacteria Lactobacillus spp. and other Lactobacillaceae genera were enriched only in DCM foals, specifically during their second and third week of life. Predicted microbiome functions estimated computationally suggested that SFM foals had higher mean sequence counts for taxa contributing to the digestion of lipids, simple and complex carbohydrates, and protein. -

Susceptibility of Archaea to Antimicrobial Agents: Applications to Clinical Microbiology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REVIEW 10.1111/j.1469-0691.2012.03913.x Susceptibility of archaea to antimicrobial agents: applications to clinical microbiology S. Khelaifia and M. Drancourt Unite´ de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, UMR CNRS 6236 IRD 3R198, Me´diterrane´e Infection, Faculte´ de Me´decine, Aix-marseille-Universite´, Marseille, France Abstract We herein review the state of knowledge regarding the in vitro and in vivo susceptibility of archaea to antimicrobial agents, including some new molecules. Indeed, some archaea colonizing the human microbiota have been implicated in diseases such as periodontopathy. Archaea are characterized by their broad-spectrum resistance to antimicrobial agents. In particular, their cell wall lacks peptidoglycan, making them resistant to antimicrobial agents interfering with peptidoglycan biosynthesis. Archaea are, however, susceptible to the pro- tein synthesis inhibitor fusidic acid and imidazole derivatives. Also, squalamine, an antimicrobial agent acting on the cell wall, proved effective against human methanogenic archaea. In vitro susceptibility data could be used to design protocols for the decontamination of complex microbiota and the selective isolation of archaea in anaerobic culture. Keywords: Antimicrobial agent, archaea, methanogenic archaea, microbiota, susceptibility testing Article published online: 23 May 2012 Clin Microbiol Infect 2012; 18: 841–848 Corresponding author: M. Drancourt, Unite´ des Rickettsies, Fa- culte´ de Me´decine, 27, Boulevard Jean Moulin-Cedex 5, France E-mail: [email protected] Methanogenic archaea (herein referred to as methano- Introduction gens) are the sole organisms producing methane from H2 +CO2 [6]. -
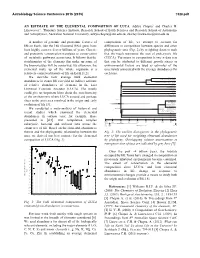
An Estimate of the Elemental Composition of Luca
Astrobiology Science Conference 2015 (2015) 7328.pdf AN ESTIMATE OF THE ELEMENTAL COMPOSITION OF LUCA. Aditya Chopra1 and Charles H. Lineweaver1, 1Planetary Science Institute, Research School of Earth Sciences and Research School of Astronomy and Astrophysics, Australian National University, [email protected], [email protected] A number of genomic and proteomic features of composition of life, we attempt to account for life on Earth, like the 16S ribosomal RNA gene, have differences in composition between species and other been highly conserved over billions of years. Genetic phylogenetic taxa (Fig. 2) by weighting datasets such and proteomic conservation translates to conservation that the result represents the root of prokaryotic life of metabolic pathways across taxa. It follows that the (LUCA). Variations in composition between data sets stoichiometry of the elements that make up some of that can be attributed to different growth stages or the biomolecules will be conserved. By extension, the environmental factors are used as estimates of the elemental make up of the whole organism is a uncertainty associated with the average abundances for relatively conserved feature of life on Earth [1,2]. each taxa. We describe how average bulk elemental Euryarchaeota 1a Methanococci, Methanobacteria, Methanopyri 7 Euryarchaeota 1b abundances in extant life can yield an indirect estimate 6 Thermoplasmata, Methanomicrobia, Halobacteria, Archaeoglobi Euryarchaeota 2 4 Thermococci of relative abundances of elements in the Last Crenarchaeota Sulfolobus, Thermoproteus ? Thaumarchaeota Universal Common Ancestor (LUCA). The results Cenarchaeum ? Korarchaeota ARMAN could give us important hints about the stoichiometry ? 2 Archaeal Richmond Mine Acidophilic Nanoorganisms Nanoarchaeota of the environment where LUCA existed and perhaps Archaea Terrabacteria clues to the processes involved in the origin and early Actinobacteria, Deinococcus-Thermus, 1 Cyanobacteria, Life Chloroflexi, 9 evolution of life [3]. -

Establishment and Analysis of Microbial Communities Capable of Producing Methane from Grass Waste at Extremely High C/N Ratio
International Journal of New Technology and Research (IJNTR) ISSN:2454-4116, Volume-2, Issue-9, September 2016 Pages 81-86 Establishment and Analysis of Microbial Communities Capable of Producing Methane from Grass Waste at Extremely High C/N Ratio S. Matsuda, T. Ohtsuki* Anaerobic digestion is a conventional method for biomass Abstract— Acclimation of microbial communities, aiming to utilization [8]. Despite the fact that a great deal of research methane production from grass as a sole substrate at extremely for methane production from grass has been conducted high carbon-to-nitrogen (C/N) ratio, was conducted. In a series especially in recent years, almost processes need supply of of experiments with various sizes of added grass, two microbial communities showing high methane production were obtained abundant sludge source such as sewage and manure [9, 10]. with powdered grass. In the two microbial communities Many studies indicated that the optimal carbon-to-nitrogen designated NR and RP, Bacteroidia including genera (C/N) ratio in methane fermentation were 25-30, and the C/N Bacteroides, Dysgonomonas, Proteiniphilum, and Alistipes were ratio higher than 40 are not generally suitable [11]. The C/N detected as dominant members in eubacteria. It was also shown ration of raw grass shows a wide range; wild grassy weed is at that Methanomicrobia and Methanobacteria including genera relatively high (>59) while lawn grass is at low (<29) [12]. Methanomassiliicoccus and Methanobacterium were found as dominant members in methanogen. It is noteworthy that Therefore, it is difficult to conduct methane fermentation nitrogen fixation were observed both in NR and RP, suggesting with wild grass-only, also being due to less degradability of that insufficiency of nitrogen sources would be complemented lignocellulose [13]. -

Brian W. Waters
INVESTIGATION OF 2-OXOACID OXIDOREDUCTASES IN METHANOCOCCUS MARIPALUDIS AND LARGE-SCALE GROWTH OF M. MARIPALUDIS by BRIAN W. WATERS (Under the direction of Dr. William B. Whitman) ABSTRACT With the emergence of Methanococcus maripaludis as a genetic model for methanogens, it becomes imperative to devise inexpensive yet effective ways to grow large numbers of cells for protein studies. Chapter 2 details methods that were used to cut costs of growing M. maripaludis in large scale. Also, large scale growth under different conditions was explored in order to find the conditions that yield the most cells. Chapter 3 details the phylogenetic analysis of 2-oxoacid oxidoreductase (OR) homologs from M. maripaludis. ORs are enzymes that catalyze the oxidative decarboxylation of 2-oxoacids to their acyl-CoA derivatives in many prokaryotes. Also in chapter 3, a specific OR homolog in M. maripaludis, an indolepyruvate oxidoreductase (IOR), was mutagenized, and the mutant was characterized. PCR and Southern hybridization analysis showed gene replacement. A no-growth phenotype on media containing aromatic amino acid derivatives was found. INDEX WORDS: Methanococcus maripaludis, fermentor, 2-oxoacid oxidoreductase, indolepyruvate oxidoreductase INVESTIGATION OF 2-OXOACID OXIDOREDUCTASES IN METHANOCOCCUS MARIPALUDIS AND LARGE-SCALE GROWTH OF M. MARIPALUDIS by BRIAN W. WATERS B.S., The University of Georgia,1999 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2002 © 2002 Brian W. Waters All Rights Reserved INVESTIGATION OF 2-OXOACID OXIDOREDUCTASES IN METHANOCOCCUS MARIPALUDIS AND LARGE-SCALE GROWTH OF M. MARIPALUDIS by BRIAN W. -

1 Development of Prediction Models of Methane Production by Sheep
Development of Prediction Models of Methane Production by Sheep and Cows Using Rumen Microbiota Data Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Boyang Zhang Graduate Program in Animal Sciences The Ohio State University 2018 Master's Examination Committee: Dr. Zhongtang Yu, Advisor Dr. Moraes, Co-advisor Dr. Firkins 1 Copyrighted by Boyang Zhang 2018 2 Abstract Methane emission from the rumen leads to approximately 10 % loss of the ingested energy, at the conversion of digestible to metabolizable energy. Besides research on mitigation of methane emission, much research interests have also been gravitated towards development of prediction models of methane emissions from livestock because the global warming is reducing agriculture productivity (Johnson and Johnson, 1995). An accurate prediction of enteric methane production from cattle and sheep can assist in balancing the increased livestock production with subsequent environmental impacts. Methane is an inevitable byproduct of the microbial fermentation processes in the rumen, and certain ruminal microbes have direct impacts to methane production (Morgavi et al., 2010). Thus, we hypothesized that the inclusion of individual microbial groups as predictor variables could improve the robustness and accuracy of prediction models. However, inclusion of microbial variables into prediction models can result in overfitting. Machine-learning algorithms can automatically select the key explanatory predictors, and Linear Mixed Models can provide a framework to predict random effects describing between animal variations. We proposed three novel frameworks for subset selections of microbial variables (MV) and one framework for generalized linear mixed models (GLMM) using L1-penalization (GLMMLASSO) selection with cross-validations (CV) ii to address the overfitting problems and to develop parsimonious prediction models of methane production. -

Lifestyle Preferences Drive the Structure and Diversity of Bacterial and Archaeal Communities in a Small Riverine Reservoir
www.nature.com/scientificreports OPEN Lifestyle preferences drive the structure and diversity of bacterial and archaeal communities in a small riverine reservoir Carles Borrego1,2, Sergi Sabater1,3* & Lorenzo Proia1,4 Spatial heterogeneity along river networks is interrupted by dams, afecting the transport, processing, and storage of organic matter, as well as the distribution of biota. We here investigated the structure of planktonic (free-living, FL), particle-attached (PA) and sediment-associated (SD) bacterial and archaeal communities within a small reservoir. We combined targeted-amplicon sequencing of bacterial and archaeal 16S rRNA genes in the DNA and RNA community fractions from FL, PA and SD, followed by imputed functional metagenomics, in order to unveil diferences in their potential metabolic capabilities within the reservoir (tail, mid, and dam sections) and lifestyles (FL, PA, SD). Both bacterial and archaeal communities were structured according to their life-style preferences rather than to their location in the reservoir. Bacterial communities were richer and more diverse when attached to particles or inhabiting the sediment, while Archaea showed an opposing trend. Diferences between PA and FL bacterial communities were consistent at functional level, the PA community showing higher potential capacity to degrade complex carbohydrates, aromatic compounds, and proteinaceous materials. Our results stressed that particle-attached prokaryotes were phylogenetically and metabolically distinct from their free-living counterparts, and that performed as hotspots for organic matter processing within the small reservoir. Spatial heterogeneity of river networks results from the sequence of lotic segments—which promote the transport and quick transformation of materials—and lentic segments such as large pools and wetlands—which mostly contribute to the process and storage of organic matter 1,2. -

Methanogens Diversity During Anaerobic Sewage Sludge Stabilization and the Effect of Temperature
processes Article Methanogens Diversity during Anaerobic Sewage Sludge Stabilization and the Effect of Temperature Tomáš Vítˇez 1,2, David Novák 3, Jan Lochman 3,* and Monika Vítˇezová 1,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] 2 Department of Agricultural, Food and Environmental Engineering, Faculty of AgriSciences, Mendel University, 61300 Brno, Czech Republic 3 Department of Biochemistry, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] * Correspondence: [email protected] (J.L.); [email protected] (M.V.); Tel.: +420-549-495-602 (J.L.); Tel.: +420-549-497-177 (M.V.) Received: 29 June 2020; Accepted: 10 July 2020; Published: 12 July 2020 Abstract: Anaerobic sludge stabilization is a commonly used technology. Most fermenters are operated at a mesophilic temperature regime. Modern trends in waste management aim to minimize waste generation. One of the strategies can be achieved by anaerobically stabilizing the sludge by raising the temperature. Higher temperatures will allow faster decomposition of organic matter, shortening the retention time, and increasing biogas production. This work is focused on the description of changes in the community of methanogenic microorganisms at different temperatures during the sludge stabilization. At higher temperatures, biogas contained a higher percentage of methane, however, there was an undesirable accumulation of ammonia in the fermenter. Representatives of the hydrogenotrophic genus Methanoliea were described at all temperatures tested. At temperatures up to 50 ◦C, a significant proportion of methanogens were also formed by acetoclastic representatives of Methanosaeta sp. and acetoclastic representatives of the order Methanosarcinales.