9180 Art 18.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Plant Growth Regulators for Branching of Nursery Trees in NY State Mario Miranda Sazo1 and Terence L
The Use of Plant Growth Regulators for Branching of Nursery Trees in NY State Mario Miranda Sazo1 and Terence L. Robinson2 1Cornell Cooperative Extension, Lake Ontario Fruit Program, Newark, NY 2Department of Horticulture, Cornell University, Geneva, NY he quality of nursery trees has a large impact on early pro- the use of Maxcel has been adopted by Italian nurserymen and is a duction and profitability of high density systems. Today, key step for the successful production of well feathered “knip-boom” nurserymen are not only asked to produce trees of good cali- apple trees. Depending on cultivar, Italian nurserymen apply from 1 T per but also highly to 4 Maxcel treatments with spray intervals of 5-7 days depending A new plant growth regulator, cyclanilide branched trees for on temperatures after application (Dr. Walter Guerra, Italy, personal “ the Tall Spindle communication). Since 2009, a new branching agent, cyclanilide (Tradename=Tiberon) very effectively system with short, (Tiberon formulated by Bayer Environmental Science, N.C. USA), induced lateral branching in nursery well positioned lat- has been available in the US for use in outdoor nurseries of apple, trees of 4 apple cultivars in the warm eral branches with sweet cherry, pear, and plum in Florida, Idaho, Oregon, Michigan, and humid year of 2010 in NY State. wide crotch angles. and Washington States. It is not currently registered for tree fruit This has required nursery use in New York State, Europe, or elsewhere. However, it also stopped shoot growth nurseries to im- Studies of the effects of Tiberon for branch induction of nursery for several weeks and reduced final prove their man- apple trees (and other fruit species) were conducted by Dr. -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

Cosmic Crisp's Growth Is out of This World
- Advertisement - Cosmic Crisp's growth is out of this world 1 / 2 April 26, 2021 After a record-breaking sophomore year that hasn't finished, the Cosmic Crisp apple’s growth trajectory indicates it will launch to the top of the apple chart in no time. Nielsen data show monumental growth, reaching No. 11 in sales dollars in March, and No. 14 over the 52 weeks ending March 27. If retailers do not have a Cosmic Crisp apple program in place now, they are missing out on very important sales and customer expectations. “Cosmic Crisp volume is planned to more than double this fall, and double again for the 2022 harvest. This volume will rival current core variety volume, displacing mature varieties that are being replaced with higher color strains and higher flavor varieties,” said Catherine Gipe-Stewart, communications manager. “Cosmic Crisp is rising so quickly, it has potential to become a top-five variety at this time next year.” Nielsen data show a 595 percent increase in dollars and a 720 percent in volume over the four weeks ending March 27. In March alone, Cosmic Crisp earned the 11th spot with $4.3 million in sales and 1.8 million pounds, at an average price of $2.52 per pound, right in line with Honeycrisp at $2.55 per pound. Looking at a yearly perspective (last 52 weeks), Cosmic Crisp has earned $20 million in sales, with 7.7 million pounds. “Historically, apples like Cosmic Crisp take root in the Pacific region, and spread eastward like wildfire,” said Gipe-Stewart. -
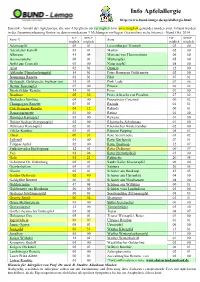
02 Apfelallergie Sortenliste Allgemein
Info Apfelallergie http://www.bund-lemgo.de/apfelallergie.html Statistik - Anzahl der Apfelsorten, die von Allergikern als verträglich bzw. unverträglich gemeldet worden sind. Erfasst werden in der Zusammenfassung Sorten zu denen mindestens 3 Meldungen vorliegen. Gesamtliste siehe Internet - Stand Okt. 2014 ver- unver- ver- unver- Sorte *) Sorte träglich träglich träglich träglich Adamsapfel 05 01 Luxemburger Triumph 03 00 Adersleber Kalvill 03 01 Martini 02 00 Alkmene 41 04 Minister von Hammerstein 06 00 Ananasrenette 06 01 Mutterapfel 05 00 Apfel aus Croncels 03 00 Notarisapfel 08 00 Arlet 02 01 Ontario 12 00 Altländer Pfannkuchenapfel 14 01 Peter Heusgens Goldrenette 02 00 Baumanns Renette 01 01 Pilot 07 01 Berlepsch, Goldrenette Freiherr von 35 03 Pink Lady 07 06 Berner Rosenapfel 07 00 Pinova 06 02 Biesterfelder Renette 14 01 Piros 01 00 Braeburn 05 32 Prinz Albrecht von Preußen 27 02 Brettacher Sämling 04 00 Purpurroter Cousinot 00 02 Champagner Renette 07 03 Reanda 01 01 Cox Orangen-Renette 04 12 Relinda 00 01 Damasonrenette 03 00 Retina 01 00 Danziger Kantapfel 05 00 Rewena 01 00 Doktor Seeligs Orangenapfel 02 00 Rheinische Schafsnase 01 00 Dülmener Rosenapfel 02 01 Rheinischer Winterrambur 02 00 Eifeler Rambur 03 01 Ripston Pepping 05 01 Elstar 09 31 Rote Sternrenette 03 02 Fallstaff 01 00 Roter Berlepsch 07 01 Filippas Apfel 02 00 Roter Boskoop 32 07 Finkenwerder Herbstprinz 12 01 Roter Delicious 00 07 Fuji 01 06 Roter Herbstkalvill 03 00 Gala 01 21 Rubinette 20 04 Geheimrat Dr. Oldenburg 02 03 Sankt Galler Klosterapfel 06 00 -

Consumer Evaluation of New, Antique, and Little Known Apple Varieties
Consumer Evaluation of New, Antique, and Little Known Apple Varieties Duane W. Greene and Jon M. Clements Department of Plant, Soil, and Insect Sciences, University of Massachusetts Introduction for them. Honeycrisp is an example of an apple that not only has become extremely popular, but Massachusetts has had a long history of apple Massachusetts appears to be a favorable place to grow production. Due to its favorable climate, it has been a this apple. leading producer in the United State of the variety In the past, private breeders, University breeding McIntosh, along with Maine, Vermont, and New York. programs, and nurseries received compensation for Over the past few years, the dynamics of apple patented varieties by receiving royalties from the sale production has changed dramatically due in large part of trees. Because apple breeding programs are very to the rapid expansion of apple production overseas, expensive to operate, the royalties received were especially in the southern hemisphere and a logarithmic insufficient to cover the cost to maintain a breeding increase in production in China. While Massachusetts program. Undoubtedly, new varieties will be released has a climate that favors the production of high quality in the future in an entirely different way. Tree sales, apples, return to growers has declined steadily over production, and marketing of the best and most the past 20 years. Other geographic locations can promising varieties will be under the strict control of produce nearly twice as many apples per acre because patent holders. Trees will be sold only to large growers of high light conditions, a long growing season, and willing to sign agreements, and they will probably be abundant water. -
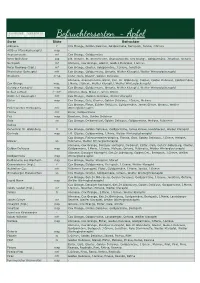
Befruchtersorten
Befruchtersorten - Äpfel Sorte Blüte Befruchter Alkmene f Cox Orange, Golden Deicious, Goldparmäne, Berlepsch, Jamba, J.Grieve Altläner Pfannkuchenapfel msp - Ananasrenette mf Cox Orange, Goldparmäne Roter Bellefleur ssp z.B. Ontario, Rt. Sternrenette, Ananasrenette, Cox Orange, Goldparmäne, Jonathan, Ontario Berlepsch mf Alkmene, Cox Orange, Gloster, Golden Delicious, J.Grieve Roter Boskoop (tripl.) mf Cox Orange, Gloster, Goldparmäne, J.Grieve, Jonathan Rheinischer Bohnapfel mfr Cox Orange, Goldparmäne, Ontario, Weißer Klarapfel, Weißer Winterglockenapfel Braeburn m-sp Elstar, Gala, Gloster, Golden Delicious Alkmene, Ananasrenette, Elstar, Geh. Dr. Oldenburg, Gloster, Golden Delicious, Goldparmäne, Cox Orange msp. I.Marie, J.Grieve, Weißer Klarapfel, Weißer Winterglockenapfel Danziger Kantapfel msp Cox Orange, Goldparmäne, Ontario, Weißer Klarapfel, Weißer Winterglockenapfel Delbar Estivale f - mfr Alkmene, Gala, Gloster, James Grieve Dülmener Rosenapfel mfr Cox Orange, Golden Delicious, Weißer Klarapfel Elstar msp Cox Orange, Gala, Gloster, Golden Delicious, J.Grieve, Melrose Cox Orange, Elstar, Golden Delicious, Goldparmäne, James Grieve, Ontario, Weißer Finkenwerder Herbstprinz mfr Winterglockenapfel Florina mfr Elstar, Goldparmäne Fuji msp Braeburn, Gala, Golden Delicious Gala sp Cox Orange, Delbarestival, Golden Delicious, Goldparmäne, Melrose, Rubinette Galiwa - - Geheimrat Dr. Oldenburg fr Cox Orange, Golden Delicious, Goldparmäne, James Grieve, Landsberger, Weißer Klarapfel Gerlinde msp z.B. Gloster, Goldparmäne, I.Marie, Weißer -

Small Steps to a Big Future for Massachusetts Cider
Small Steps to a Big Future for Massachusetts Cider Apples Elizabeth Garofalo and Jon Clements University of Massachusetts In Massachusetts, our love aff air with cider has a long and illustrious -- if sometimes notorious -- history dĂďůĞϭ͘ůĞǀĞŶĐƵůƚŝǀĂƌƐŐƌĂĨƚĞĚŽŶƚŽŽůĚĞƌƚƌĞĞƐĂƚƚŚĞ that predates even John Chapman (AKA Johnny Ap- hDĂƐƐŽůĚ^ƉƌŝŶŐKƌĐŚĂƌĚŝŶĞůĐŚĞƌƚŽǁŶ͕D͘ pleseed). In recent decades, there has been a growing ĞƐĐƌŝƉƚŝŽŶƐĂƌĞŐĞŶĞƌĂů͘ŚĂƌĂĐƚĞƌŝƐƚŝĐƐŽĨĞĂĐŚ nationwide passion for the fermented beverage enjoyed ǀĂƌŝĞƚLJǁŝůůďĞĞǀĂůƵĂƚĞĚĨŽƌDƉƌŽĚƵĐƚŝŽŶĐŽŶĚŝƚŝŽŶƐ͘ by our forebears. Unfortunately, there is a dearth in ƵůƚŝǀĂƌ &ůĂǀŽƌWƌŽĨŝůĞ production of desirable apples for traditional hard cider. ůŬŵĞŶĞ;ĂŬĂĂƌůLJtŝŶĚƐŽƌͿ ^ǁĞĞƚͲƐŚĂƌƉ (Re ferred to as just cider from here on, as it should ƐŚŵĞĂĚ͛Ɛ<ĞƌŶĞů ^ǁĞĞƚͲƐŚĂƌƉ be). This has led to a market fl ooded with a bevy of ŽƵƌƚWĞŶĚƵWůĂƚ ^ǁĞĞƚͲƚĂƌƚ apple-based adult cider beverages possessing less than ŐƌĞŵŽŶƚZƵƐƐĞƚƚ ^ǁĞĞƚͲƐŚĂƌƉ ůůŝƐŝƚƚĞƌ ŝƚƚĞƌƐǁĞĞƚ traditional qualities. There are some orchards in the &ŽdžǁŚĞůƉ ŝƚƚĞƌƐŚĂƌƉ Northeast that have been making positive headway in <ŝŶŐƐƚŽŶůĂĐŬ ŝƚƚĞƌƐŚĂƌƉ increasing traditional cider apple plantings. There re- DĞĚĂŝůůĞΖKƌ ŝƚƚĞƌƐǁĞĞƚ mains, however, a chronic shortage of traditional cider DŝĐŚĞůŝŶ ŝƚƚĞƌƐǁĞĞƚ apples (Fabien-Ouellet & Conner, 2017). This project ZĞĚĨŝĞůĚ ŝƚƚĞƌƐǁĞĞƚ aims to provide Massachusetts growers with informa- ^ƚ͘ĚŵƵŶĚ͛ƐZƵƐƐĞƚ ^ǁĞĞƚ tion specifi c to Massachusetts cider apple varieties that contribute to a quality top-shelf cider. Not all apple varieties are created equally. Some susceptibility. But, with craft cideries attempting to apples are far better suited for fresh eating and baking. distinguish themselves from mass-produced sweet- The supermarket is fi lled with varieties we all know tasting ciders often made from apple juice concentrate, and love: McIntosh, Honeycrisp, Fuji, and Gala. These there is an opportunity for local growers to fi nd a new varieties do not possess the characteristics necessary and exciting niche for their apples (Raboin, 2017). -

INF03 Reduce Lists of Apple Varieites
ECE/TRADE/C/WP.7/GE.1/2009/INF.3 Specialized Section on Standardization of Fresh Fruit and Vegetables Fifty-fifth session Geneva, 4 - 8 May 2009 Items 4(a) of the provisional agenda REVISION OF UNECE STANDARDS Proposals on the list of apple varieties This note has been put together by the secretariat following the decision taken by the Specialized Section at its fifty-fourth session to collect information from countries on varieties that are important in international trade. Replies have been received from the following countries: Canada, Czech Republic, Finland, France, Germany, Italy, Netherlands, New Zealand, Poland, Slovakia, South Africa, Sweden, Switzerland and the USA. This note also includes the documents compiled for the same purpose and submitted to the fifty-second session of the Specialized Section. I. Documents submitted to the 52nd session of the Specialized Section A. UNECE Standard for Apples – List of Varieties At the last meeting the 51 st session of the Specialized Section GE.1 the delegation of the United Kingdom offered to coordinate efforts to simplify the list of apple varieties. The aim was to see what the result would be if we only include the most important varieties that are produced and traded. The list is designed to help distinguish apple varieties by colour groups, size and russeting it is not exhaustive, non-listed varieties can still be marketed. The idea should not be to list every variety grown in every country. The UK asked for views on what were considered to be the most important top thirty varieties. Eight countries sent their views, Italy, Spain, the Netherlands, USA, Slovakia, Germany Finland and the Czech Republic. -

Stemilt PLU Numbers - Apples
Stemilt PLU Numbers - Apples Variety PLU RSS Braeburn 4103 00741839041030 Large Braeburn 4101 00741839041016 Small Organic Braeburn 94103 00741839941033 Large Organic Braeburn 94101 00741839941019 Small Cameo 3066 00741839030669 Large Cameo 3065 00741839030652 Small Organic Cameo 93066 00741839930662 Large Organic Cameo 93065 00741839930655 Small Cosmic Crisp 3507 00741839035077 All Organic Cosmic Crisp 93507 00741839935070 All Fuji 4131 00741839041313 Large Fuji 4129 00741839041290 Small Organic Fuji 94131 00741839941316 Large Organic Fuji 94129 00741839941293 Small Gala 4135 00741839041351 Large Gala 4133 00741839041337 Small Organic Gala 94135 00741839941354 Large Organic Gala 94133 00741839941330 Small Gingergold 4096 00741839040965 Large Gingergold 4097 00741839040972 Small Organic Gingergold 94096 00741839940968 Large Organic Gingergold 94097 00741839940975 Small Granny Smith 4017 00741839040170 Large Granny Smith 4139 00741839041399 Small Organic Granny Smith 94017 00741839940173 Large Organic Granny Smith 94139 00741839941392 Small Jonagold 4147 00741839041474 Large Jonagold 4145 00741839041450 Small Organic Jonagold 94147 00741839941477 Large Organic Jonagold 94145 00741839941453 Small Pink Lady 4130 00741839041306 Large Pink Lady 4128 00741839041283 Small Organic Pink Lady 94130 00741839941309 Large Organic Pink Lady 94128 00741839941286 Small Rave 3487 00741839034872 All Organic Rave 93487 00741839934875 All Rome 4172 00741839041726 Large Rome 4170 00741839041702 Small Organic Rome 94172 00741839941729 Large Organic Rome 94170 -

Cosmic Crisp 74183935002-6 Gala
CONVENTIONAL APPLES COSMIC CRISP 74183935002-6 2lb GALA 74183900352-6 HONEYCRISP 74183900351-9 PINATA 74183900350-2 PINK LADY 74183900354-0 RAVE 74183900353-3 SWEETANGO 74183900718-0 ORGANIC APPLES ORGANIC COSMIC CRISP 74183936002-5 2lb ORGANIC FUJI 74183900430-1 ORGANIC GALA 74183900431-8 ORGANIC GRANNY SMITH 74183900432-5 ORGANIC HONEYCRISP 74183900795-1 ORGANIC PINATA 74183900438-7 ORGANIC PINK LADY 74183900433-2 ORGANIC RAVE 741839334002-7 ORGANIC RED DELICIOUS 74183900434-9 ORGANIC SWEETANGO 74183900437-0 CONVENTIONAL PEAR BARTLETT 74183900360-1 2lb BOSC 74183900362-5 DANJOU 74183900361-8 RED PEAR 74183900363-2 ORGANIC PEAR ORGANIC BARTLETT 74183900785-2 2lb ORGANIC BOSC 74183900788-3 ORGANIC CONCORD 74183900728-9 ORGANIC DANJOU 74183900786-9 ORGANIC RED PEAR 74183900787-6 ORGANIC TOSCA 74183900732-6 CONVENTIONAL APPLE 3lb COSMIC CRISP 74183935003-3 FUJI 74183900588-9 GALA 74183900590-2 GOLDEN DELICIOUS - Use Up 74183900724-1 GRANNY SMITH 74183900591-9 HONEYCRISP 74183900592-6 PINATA 74183900594-0 PINK LADY 74183900593-3 RED DELICIOUS - Use Up 74183900723-4 SWEETANGO 74183900675-6 ORGANIC APPLE ORGANIC FUJI 74183900725-8 3lb ORGANIC GALA 74183900596-4 ORGANIC GRANNY SMITH 74183900597-1 ORGANIC HONEYCRISP 74183900722-7 ORGANIC PINATA 74183900599-5 ORGANIC PINK LADY 74183900598-8 CONVENTIONAL PEAR BOSC 74183900720-3 3lb RED PEAR 74183900719-7 BARTLETT 74183900714-2 CONCORD 74183900710-4 DANJOU 74183900712-8 TOSCA - Use Up 74183900715-9 CONVENTIONAL APPLE COSMIC CRISP 74183900676-3 4lb GALA 74183900741-8 HONEYCRISP 74183900677-0 PINATA -

Apple and Pear Production in Romania
Universitatea de Ştiinţe Agronomice şi Medicină Veterinară Facultatea de Horticultură – București Apple and Pear production in Romania Prof. dr. Florin STĂNICĂ Assoc. Prof. dr. Adrian ASĂNICĂ Universitatea de Științe Agronomice şi Medicină Veterinară București EUFRIN WG Meeting - Balandran – 3rd of March 2017 University of Agronomic Sciences and Veterinary Medicine Bucureşti B-dul Mărăşti, 59, 011464, Bucureşti, www.usamv.ro Faculty of Horticulture Research Center for Integrated Fruit Growing B-dul Mărăşti, nr 59, Sector 1, 011464, Bucureşti Tel. +40.722.641795, Fax : +40.21.3182888, www.pomosat.ro E-mail: [email protected] Introduction Position: 43˚ 37’ and 48˚15’ northern latitude and 20 ˚15’ and 29˚41’ eastern longitude Area: 238,392 km2 Population: aprox . 22,500,000 Language: Romanian (neo-latin) Climate: - temperate, excessive continental - average annual temperature: 8-11 degrees Celsius - annual rain fall: 450-800 mm Agriculture: - arable land: 10 million hectares - vineyards: 250,000 hectares: several local cv. - fruit orchards: 200,000 hectares: temperate fruits: Apples, European Plums, Pears, Quinces, Peaches, Apricots, Sweet & Sour Cherries, Walnuts, Hazelnuts, Chestnuts, Berries etc. Fruit production areas Research Center fo Integrated Fruit Growing USAMV Bucureşti Apple production Poly Scab resistant varieties since 1989: Florina, Generos, Pionier, Romus 3 Research Center fo Integrated Fruit Growing USAMV Bucureşti Apple production Scab resistant varieties since 1989: Florina, Generos, Pionier, Romus 3 Romus -

Apple Cultivars
Apple Cultivars: The newer apple cultivars that we recommend for A Geneva Perspective careful testing by growers in New York include Susan Brown and Kevin Maloney ‘Ambrosia’, ‘Braeburn’, Department of Horticultural Sciences, Cornell University, ‘Corail’, ‘Sansa’, and New York State Agricultural Experiment Station, Geneva, NY ‘Zestar’. Other apples discussed in this article This work was funded in part by the New York State may also have limited Apple Research and Development Program. opportunities depending on the grower’s market and customer acceptance. unding from the New York Apple Highland site is for assessment of disease Research and Development and pest susceptibility. The 1995 planting Fprogram (ARDP) and the New York includes the following varieties and Apple Association (NYAA) aids our advanced selections: ‘Arlet’, ‘Braeburn’ the fruits are slightly square. It has been evaluation research on the performance (control), ‘Creston’, ‘Cameo’, described as very attractive, of good size, of new cultivars and breeding selections. ‘Enterprise’, ‘Fortune’, ‘Fuji’ (BC#2), crisp, sweet, low acid, very juicy, distinct This article features some of the cultivars ‘Yataka Fuji’ (control), ‘Gala Supreme’, but mild, with a pleasant aroma. It has we have evaluated and offers information ‘Ginger Gold’, ‘Golden Delicious’ been rated well in test trials. Trees are on other cultivars that are either not (control), ‘Golden Supreme’, ‘Goldrush’, productive, upright, spur-type and available for testing or are not ‘Honeycrisp’, NY 75414-1, ‘Orin’, grower friendly. Trees should not be recommended for our region, particularly ‘Pristine’, ‘Sansa’, ‘Shizuka’, ‘Suncrisp’ overcropped early. Two harvests are in regard to having too long a growing and’‘Sunrise’.