Microneedles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide
Human Journals Research Article September 2015 Vol.:4, Issue:2 © All rights are reserved by C. Aparna et al. Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide Keywords: Nateglinide, transdermal patch and gel, HPMC, ethyl cellulose, carbopol, PVA, PVP ABSTRACT Anusha Gundeti, C. Aparna*, Dr. Prathima Srinivas The objective of the present work was to formulate Transdermal Drug Delivery systems of Nateglinide, an Department of Pharmaceutics, Sri Venkateshwara antidiabetic drug belonging to meglitinide class with a half life of 1.5 hrs. Transdermal patches containing nateglinide were College of Pharmacy, prepared by solvent casting method using the combinations Affiliated to Osmania University, of HPMC:EC, PVA:PVP, HPMC:Eudragit RS 100, Eudragit RL100:RS100 in different proportions and by incorporating Madhapur, Hyderabad, Telangana -500081, India. different permeation enhancers (polyethylene glycol 400, Su bmission: 7 September 2015 DMSO). The transdermal patches were evaluated for their physicochemical properties like thickness, weight variation, Accepted: 11 September 2015 folding endurance, percentage moisture absorption, percentage Published: 25 September 2015 moisture loss, in-vitro diffusion studies & ex-vivo permeation studies. Transdermal Gel was formulated using HPMC, carbopol 934, carbopol 940 and methyl cellulose. Gels were evaluated for homogeneity, pH, viscosity, drug content, in-vitro diffusion studies & ex-vivo permeation studies. By comparing the drug release F5 (HPMC:EC) formulation was selected as optimized formulation as it could sustain the drug release for 12 hrs i.e. 99.2% when compared to gel. Stability studies were www.ijppr.humanjournals.com carried out according to ICH guidelines and the patches maintained integrity and good physicochemical properties during the study period. -

An Archetype Swing in Transdermal Drug Delivery
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 A COMPREHENSIVE REVIEW ON MICRONEEDLES - AN ARCHETYPE SWING IN TRANSDERMAL DRUG DELIVERY G. Ravi*, N. Vishal Gupta, M. P. Gowrav Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Shri Shivarathreeshwara Nagara, Mysuru, Karnataka, India. ARTICLE INFO ABSTRACT Article history Transdermal drug delivery is the non-invasive delivery of medications through the skin Received 23/12/2016 surface into the systemic circulation. The advantage of transdermal drug delivery system is Available online that it is painless technique of administration of drugs. The advantage of transdermal drug 31/01/2017 delivery system is that it is painless technique of administration of drugs. Transdermal drug delivery system can improve the therapeutic efficacy and safety of the drugs because drug Keywords delivered through the skin at a predetermined and controlled rate. Due to the various Microneedles, biomedical benefits, it has attracted many researches. The barrier nature of stratumcorneum Hypodermic Needles, poses a danger to the drug delivery. By using microneedles, a pathway into the human body Transdermal, can be recognized which allow transportation of macromolecular drugs such as insulin or Stratumcorneum, vaccine. These microneedles only penetrate outer layers of the skin, exterior sufficient not to Patch. reach the nerve receptors of the deeper skin. Thus the microneedles supplement is supposed painless and reduces the infection and injuries. Researches from the past few years showed that microneedles have emerged as a novel carrier and considered to be effective for safe and improved delivery of the different drugs. Microneedles development is created a new pathway in the drug delivery field. -

Transdermal Nicotine Maintenance Attenuates the Subjective And
Neuropsychopharmacology (2004) 29, 991–1003 & 2004 Nature Publishing Group All rights reserved 0893-133X/04 $25.00 www.neuropsychopharmacology.org Transdermal Nicotine Maintenance Attenuates the Subjective and Reinforcing Effects of Intravenous Nicotine, but not Cocaine or Caffeine, in Cigarette-Smoking Stimulant Abusers 1 1 ,1,2 Bai-Fang X Sobel , Stacey C Sigmon and Roland R Griffiths* 1Department of Psychiatry and Behavioral Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA The effects of transdermal nicotine maintenance on the subjective, reinforcing, and cardiovascular effects of intravenously administered cocaine, caffeine, and nicotine were examined using double-blind procedures in nine volunteers with histories of using tobacco, caffeine, and cocaine. Each participant was exposed to two chronic drug maintenance phases (21 mg/day nicotine transdermal patch and placebo transdermal patch). Within each drug phase, the participant received intravenous injections of placebo, cocaine (15 and 30 mg/70 kg), caffeine (200 and 400 mg/70 kg), and nicotine (1.0 and 2.0 mg/70 kg) in mixed order across days. Subjective and cardiovascular data were collected before and repeatedly after drug or placebo injection. Reinforcing effects were also assessed after each injection with a Drug vs Money Multiple-Choice Form. Intravenous cocaine produced robust dose-related increases in subjective and reinforcing effects; these effects were not altered by nicotine maintenance. Intravenous caffeine produced elevations on several subjective ratings; nicotine maintenance did not affect these ratings. Under the placebo maintenance condition, intravenous nicotine produced robust dose-related subjective effects, with maximal increases similar to the high dose of cocaine; nicotine maintenance significantly decreased the subjective and reinforcing effects of intravenous nicotine. -

A Brief Review on Transdermal Patches
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 7 Issue 2 - June 2018 Copyright © All rights are reserved by Nidhi Sharma DOI: 10.19080/OMCIJ.2018.07.555707 A Brief Review on Transdermal Patches Nidhi Sharma* HIMT College of Pharmacy, Greater Noida, India Submission: May 12, 2018; Published: June 05, 2018 *Corresponding author: Nidhi Sharma, HIMT College of Pharmacy, Greater Noida, India, Email: Abstract healingTransdermal to an injured drug area delivery of the system body. was An presentedadvantage toof overcome a transdermal the difficulties drug delivery of drug route delivery over especiallyother types oral of route. delivery A transdermal system such patch as oral, is a topical,medicated i.v., adhesive i.m., etc. ispatch that that the patchis placed provides on the askin controlled to deliver release a specific of the dose medication of medication into the through patient, the usually skin and through into the either bloodstream. a porous It membrane promotes covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier, as a result, only medications whose molecules are small can easily penetrate the skin, so it can be delivered by this method. This review article describes the overall introduction of transdermal patches including type of transdermal patches, method of preparation of transdermal -
Transdermal Opioid Patches: Quick Reference Guide
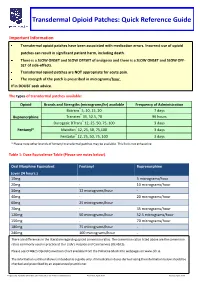
Transdermal Opioid Patches: Quick Reference Guide Important Information Transdermal opioid patches have been associated with medication errors. Incorrect use of opioid patches can result in significant patient harm, including death. There is a SLOW ONSET and SLOW OFFSET of analgesia and there is a SLOW ONSET and SLOW OFF- SET of side-effects. Transdermal opioid patches are NOT appropriate for acute pain. The strength of the patch is prescribed in micrograms/hour. If in DOUBT seek advice. The types of transdermal patches available: Opioid Brands and Strengths (micrograms/hr) available Frequency of Administration Butrans® 5, 10, 15, 20 7 days Buprenorphine Transtec® 35, 52.5, 70 96 hours Durogesic DTrans® 12, 25, 50, 75, 100 3 days Fentanyl* Matrifen® 12, 25, 50, 75,100 3 days Fentadur® 12, 25, 50, 75, 100 3 days * Please note other brands of fentanyl transdermal patches may be available. This list is not exhaustive. Table 1: Dose Equivalence Table (Please see notes below). Oral Morphine Equivalent Fentanyl Buprenorphine (over 24 hours.) 10mg 5 micrograms/hour 20mg - 10 micrograms/hour 30mg 12 micrograms/hour - 40mg - 20 micrograms/hour 60mg 25 micrograms/hour 70mg - 35 micrograms/hour 120mg 50 micrograms/hour 52.5 micrograms/hour 150mg - 70 micrograms/hour 180mg 75 micrograms/hour - 240mg 100 micrograms/hour - There are differences in the literature regarding opioid conversion ratios. The conversion ratios listed above are the conversion ratios commonly used in practice at Our Lady’s Hospice and Care Service (OLH&CS). Please see OLH&CS Opioid Conversion Chart available from the Palliative Meds Info webpages on www.olh.ie. -
Aptensio XR (Methylphenidate) Extended-Release Capsules
Aptensio XR™ (methylphenidate) – First-time authorized brand alternative • On May 5, 2020, Rhodes Pharmaceuticals launched an authorized brand alternative of its (Rhodes) branded Aptensio XR (methylphenidate) extended-release capsules. • Aptensio XR is approved for the treatment of attention deficit hyperactivity disorder (ADHD) in patients 6 years and older. • Methylphenidate is also available generically as an oral solution, extended-release capsule, extended-release tablet, tablet, and chewable tablet. It is also available as a brand extended-release capsule (Adhansia XR™, Jornay PM™), extended-release orally disintegrating tablet (Contempla-XR ODT™), transdermal patch (Daytrana®), extended-release chewable tablet (QuilliChew XR®), extended-release oral suspension (Quillivant XR®), and extended-release tablet (Relexxii™). — Methylphenidate oral solution, tablet, chewable tablet and extended-release tablets are approved to treat ADHD and narcolepsy. — Methylphenidate extended-release capsules, Adhansia XR, Contempla-XR ODT, Daytrana, Jornay PM, QuilliChew XR, Quillivant XR and Relexxii are approved to treat ADHD. • Aptensio XR carries a boxed warning for abuse and dependence. optumrx.com OptumRx® specializes in the delivery, clinical management and affordability of prescription medications and consumer health products. We are an Optum® company — a leading provider of integrated health services. Learn more at optum.com. All Optum® trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their respective owners. This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written consent of OptumRx. RxNews® is published by the OptumRx Clinical Services Department. ©2020 Optum, Inc. All rights reserved. . -

Transdermal and Transbuccal Drug Delivery
TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS by LONSHENG HU A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Pharmaceutical Science written under the direction of Bozena Michniak-Kohn and approved by ________________________ ________________________ ________________________ ________________________ New Brunswick, New Jersey October, 2010 ABSTRACT OF THE DISSERTATION TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS By LONSHENG HU Dissertation Director: Professor Bozena Michniak-Kohn Transdermal and transbuccal routes offer attractive alternatives for systemic delivery of drugs due to their distinct advantages: non-invasive, avoidance of first-pass effect, improved bioavailability and reduction of systemic side effects. However, only a few drugs have been successfully delivered into blood stream to reach therapeutic levels without causing notable skin irritation or damage. Transbuccal drug delivery systems are still at research stage. The major barriers to transdermal and transbuccal drug delivery are stratum corneum of skin and epithelium of buccal tissue. The objective of this work was to overcome these barriers to significantly enhance transdermal and transbuccal delivery of hydrophilic drugs without causing major damage to skin and buccal tissue. In this work, iontophoresis, chemical -

Transdermal and Parenteral Fentanyl Dosage Calculations and Conversions
CHAPTER 5 Transdermal and Parenteral Fentanyl Dosage Calculations and Conversions OBJECTIVES INTRODUCTION After reading this chapter and completing Fentanyl is a synthetic pure mu opioid all practice problems, the participant will agonist with pharmacologic properties be able to: similar to morphine, hydromorphone, 1. Describe the pharmacokinetics of oxycodone and other opioids. Important transdermal fentanyl, and variables differences about fentanyl include its that can influence dosing. high degree of potency (about 75–100 times more potent than morphine on a 2. Recommend an appropriate dose of mg-to-mg basis), and greater lipid solubil- transdermal fentanyl when switching ity than morphine. The lipophilic nature from other opioids, including rescue of fentanyl facilitates rapid diffusion opioid dosing. The participant will be across the blood-brain barrier, resulting able to describe the appropriate tim- in a quick onset of action once the drug ing of this conversion. is absorbed from the administration site. 3. Recommend a strategy for switching Fentanyl is available in several dosage from transdermal fentanyl to another formulations, and may be administered opioid regimen, including dosing and by the following routes for a variety of appropriate timing. pain-related indications: 4. Describe how to transition between n Parenteral—fentanyl may be given by intravenous (IV) fentanyl and trans- intravenous (IV) injection, IV infu- dermal fentanyl. sion, subcutaneous (SQ) infusion or intramuscular (IM) injection (although we already agreed that an IM analge- sic is an oxymoron, and this practice is discouraged). It is used parenterally preoperatively, intraoperatively and postoperatively, and is occasionally used for the management of severe acute and chronic pain in other clinical situations. -

Package Leaflet: Information for the User EVRA 203 Micrograms/24
Package leaflet: Information for the user EVRA 203 micrograms/24 hours + 33.9 micrograms/24 hours transdermal patch norelgestromin/ethinyl estradiol Important things to know about combined hormonal contraceptives (CHCs): - They are one of the most reliable reversible methods of contraception if used correctly. - They slightly increase the risk of having a blood clot in the veins and arteries, especially in the first year or when restarting a combined hormonal contraceptive following a break of 4 or more weeks. - Please be alert and see your doctor if you get symptoms of a blood clot (see section 2 “Blood clots”). Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor, pharmacist or nurse. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them. - If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What EVRA is and what it is used for 2. What you need to know before you use EVRA 3. How to use EVRA 4. Possible side effects 5. How to store EVRA 6. Contents of the pack and other information 1. What EVRA is and what is it used for EVRA contains two types of sex hormones, a progestogen called norelgestromin and an oestrogen called ethinyl estradiol. -

A Fully Integrated Microneedle-Based Transdermal Drug Delivery System
A Fully Integrated Microneedle-based Transdermal Drug Delivery System Niclas Roxhed MICROSYSTEM TECHNOLOGY LABORATORY SCHOOL OF ELECTRICAL ENGINEERING ROYAL INSTITUTE OF TECHNOLOGY ISBN 978-91-7178-751-4 ISSN 1653-5146 TRITA-EE 2007:046 Submitted to the School of Electrical Engineering KTH—Royal Institute of Technology, Stockholm, Sweden, in partial fulfillment of the requirements for the degree of Doctor of Philosophy Stockholm 2007 ii A Fully Integrated Microneedle-based Transdermal Drug Delivery System The left picture on the front cover shows an integrated microneedle-based drug delivery system fabricated and used for experiments by the author. An array of microneedles is located on the other side of the device. The right picture on the cover shows a magnified view of these side-opened hollow microneedles, likewise fabricated by the author. The needles are designed to penetrate skin tissue using a low insertion force. The needles are fabricated by deep reactive ion etching of silicon and the length of the needles is 400 μm. Copyright 2007 by Niclas Roxhed All rights reserved to the summary part of this thesis, including all pictures and figures. No part of this publication may be reproduced or transmitted in any form or by any means, without prior permission in writing from the copyright holder. The copyrights for the appended journal papers belong to the publishing houses of the journals concerned. The copyrights for the appended manuscripts belong to their authors. Printed by Universitetsservice US AB, Stockholm 2007. Thesis for the degree of Doctor of Philosophy at the Royal Institute of Technology, Stockholm, Sweden, 2007. -

Safety Page Transdermal Patches: High Risk for Error?
Safety Page Transdermal patches: High risk for error? Although transdermal patches that the patches are not being derm, there is potential for confusion, provide a useful alternative to oral applied appropriately. which may result in the patches being medications, patch administration can •Why can’t you tape it on? The technol- applied to the wrong area. be complicated. Transdermal patches ogy of most patches is designed to use Errors have been reported wherein are a common route of administration the occlusive dressing to facilitate the patients receive or apply multiple for hormonal therapy, narcotic analge- absorption of the drug through the patches at once. One man did not sia, and nicotine. There are patches skin. Some patients do not realize that survive after his wife applied six fen- available for over-the-counter and pre- the patch must be applied directly to tanyl patches to his skin at one time. scription-only use. the skin. There was a report of a Another common problem is that the Medication errors with patches patient who applied his new patch old patch is not removed when the occur in every healthcare practice set- directly on top of the old one. This new patch is applied. ting—patients’ homes, physician continued until he had four patches Clear patches have become popular offices, intensive care units, cardiac stuck to one another instead of to his because you cannot see them on the step-down units, day care facilities, skin. In one case, a practitioner skin; however, this feature has also inpatient institutional settings, emer- applied the overlay to the patient’s made them error-prone. -

FIDA Quantity Limit List 508
Elderplan 2016 Formulary Quantity Limits Drug Name Dose Form Quantity Limit Amount Quantity Limit Days acetaminophen-codeine #2 tab 300-15 ORAL TABLET 400 30 mg acetaminophen-codeine #3 tab 300-30 ORAL TABLET 400 30 mg acetaminophen-codeine #4 tab 300-60 ORAL TABLET 400 30 mg acetaminophen-codeine solution 120-12 SOLUTION 5000 30 mg/5ml acetaminophen-codeine tab 300-15 mg ORAL TABLET 400 30 acetaminophen-codeine tab 300-30 mg ORAL TABLET 400 30 acetaminophen-codeine tab 300-60 mg ORAL TABLET 400 30 acyclovir ointment 5 % TOPICAL OINTMENT 15 15 ADVAIR DISKUS AER POW BA 100 DRY POWDER INHALER 60 30 50 MCG/DOSE ADVAIR DISKUS AER POW BA 250 DRY POWDER INHALER 60 30 50 MCG/DOSE ADVAIR DISKUS AER POW BA 500 DRY POWDER INHALER 60 30 50 MCG/DOSE ADVAIR HFA AEROSOL 115-21 METERED DOSE INHALER 12 30 MCG/ACT ADVAIR HFA AEROSOL 230-21 METERED DOSE INHALER 12 30 MCG/ACT ADVAIR HFA AEROSOL 45-21 METERED DOSE INHALER 12 30 MCG/ACT H8029_2016 Formulary ID: 16200 Version 7 Last Updated: 08/19/2015 Effective Date: 01/01/2016 *Brand name drugs are capitalized, generic drugs are lower-case and italicized. Elderplan 2016 Formulary Quantity Limits Drug Name Dose Form Quantity Limit Amount Quantity Limit Days ALINIA RECON SUSP 100 MG/5ML ORAL SUSPENSION 180 30 ALINIA TAB 500 MG ORAL TABLET 6 3 ALOXI 0.075 MG/1.5ML INJECTABLE SOLUTION 5 7 ALOXI SOLUTION 0.25 MG/5ML INJECTABLE SOLUTION 5 7 AMITIZA CAP 24 MCG ORAL CAPSULE 60 30 AMITIZA CAP 8 MCG ORAL CAPSULE 60 30 amlodipine besy-benazepril hcl cap 10 ORAL CAPSULE 30 30 20 mg amlodipine besy-benazepril hcl