A Fully Integrated Microneedle-Based Transdermal Drug Delivery System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide
Human Journals Research Article September 2015 Vol.:4, Issue:2 © All rights are reserved by C. Aparna et al. Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide Keywords: Nateglinide, transdermal patch and gel, HPMC, ethyl cellulose, carbopol, PVA, PVP ABSTRACT Anusha Gundeti, C. Aparna*, Dr. Prathima Srinivas The objective of the present work was to formulate Transdermal Drug Delivery systems of Nateglinide, an Department of Pharmaceutics, Sri Venkateshwara antidiabetic drug belonging to meglitinide class with a half life of 1.5 hrs. Transdermal patches containing nateglinide were College of Pharmacy, prepared by solvent casting method using the combinations Affiliated to Osmania University, of HPMC:EC, PVA:PVP, HPMC:Eudragit RS 100, Eudragit RL100:RS100 in different proportions and by incorporating Madhapur, Hyderabad, Telangana -500081, India. different permeation enhancers (polyethylene glycol 400, Su bmission: 7 September 2015 DMSO). The transdermal patches were evaluated for their physicochemical properties like thickness, weight variation, Accepted: 11 September 2015 folding endurance, percentage moisture absorption, percentage Published: 25 September 2015 moisture loss, in-vitro diffusion studies & ex-vivo permeation studies. Transdermal Gel was formulated using HPMC, carbopol 934, carbopol 940 and methyl cellulose. Gels were evaluated for homogeneity, pH, viscosity, drug content, in-vitro diffusion studies & ex-vivo permeation studies. By comparing the drug release F5 (HPMC:EC) formulation was selected as optimized formulation as it could sustain the drug release for 12 hrs i.e. 99.2% when compared to gel. Stability studies were www.ijppr.humanjournals.com carried out according to ICH guidelines and the patches maintained integrity and good physicochemical properties during the study period. -

(12) United States Patent (10) Patent No.: US 7.427.405 B2 Agrawal Et Al
USOO7427405B2 (12) United States Patent (10) Patent No.: US 7.427.405 B2 Agrawal et al. (45) Date of Patent: Sep. 23, 2008 (54) IMMUNOSTIMULATORY Tokunaga et al., “Antitumor Activity of Deoxyribonucleic Acid Frac OLGONUCLEOTDE MULTIMERS tion from Mycobaterium bovis BCG. I. Isolation. Physicochemical Characterization, and Antitumor Activity”. J. Natl. Cancer Inst. 72: (75) Inventors: Sudhir Agrawal, Shrewsbury, MA (US); 955-962(1984). Pisetsky et al., “Stimulation of in vitro proliferation of murine lym Ekambar Kandimala, Southboro, MA phocytes by synthetic oligodeoxynucleotides'. Molecular Biology (US); Dong Yu, Westboro, MA (US) Reports 18:217-221 (1993). Krieg et al., “CpG motifs in bacterial DNA trigger direct B-cell (73) Assignee: Idera Pharmaceuticals, Inc., activation”, Nature 374: 546-549 (1995). Cambridge, MA (US) Sato et al., “Immunostimulatory DNA Sequence Necessary for Effective Intradermal Gene Immunization', Science 273: 352-354 (*) Notice: Subject to any disclaimer, the term of this (1996). patent is extended or adjusted under 35 Krieg et al., “CpG Motifs in Bacterial DNA and their Immune U.S.C. 154(b) by 209 days. Effects”, Annu. Rev. Immunol. 20: 709-760 (2002). Dalpke et al., “Immunopharmacology of CpG DNA'. Biol. Chem. (21) Appl. No.: 11/174,282 383: 1491-1500 (2002). Kandimala et al., “Towards Optimal Design of Second-Generation (22) Filed: Jul. 1, 2005 Immunomodulatory Oligonucleotides'. Curr. Opin. Mol. Ther. 4(2): 122-129 (2002). (65) Prior Publication Data Kandimala et al., “Immunomers-novel 3'-3'-Linked CpG Oligodeoxyribonucleotides as Potent Immunomodulatory Agents'. US 2006/OO19919 A1 Jan. 26, 2006 Nucleic Acids Res. 30: 4460-4469 (2002). Kandimala et al. -

Journal of Arthroscopy and Joint Surgery 6 (2019) 98E102
Journal of Arthroscopy and Joint Surgery 6 (2019) 98e102 Contents lists available at ScienceDirect Journal of Arthroscopy and Joint Surgery journal homepage: www.elsevier.com/locate/jajs Comparative evaluation of periarticular infiltration of two cocktail regimens for analgesia in post-operative patients of total knee replacement * V.K. Gautam a, Ajeet Kumar a, Munisha Agarwal b, Bushu Harna a, , Rishabh Saini a, Siddharth Sharma a, Dhananjaya Sabat a a Dept. of Orthopaedics, Maulana Azad Medical College, New Delhi, India b Dept. of Anaesthesia, Maulana Azad Medical College, New Delhi, India article info abstract Article history: Purpose: To compare the efficacy of two periarticular cocktail regimens for analgesia in postoperative Received 30 April 2018 patients of total knee replacement. Received in revised form Method: This is a Randomized Control study done over the duration of 1.5 years. Twenty-five knees of 28 October 2018 either gender were selected with inclusion criteria (All osteoarthritis patients planned for TKA) and Accepted 9 November 2018 exclusion criteria (Inflammatory arthritis, patients allergic to local anaesthetic e.g. Ropivacaine, bupi- Available online 19 November 2018 vacaine, known cardiac disorder patient having AV block, arrhythmia) & divided into 2 groups. Group A was given a cocktail of Ropivacaine, adrenaline, clonidine & cefuroxime. Keywords: & Periarticular Group B was given a cocktail of bupivacaine, fentanyl, methylprednisolone cefuroxime. The preoper- Multimodal approach ative pain of the patient was assessed using VAS score. Combined spinal and epidural anaesthesia was Perioperative analgesia given using 0.5% 2 ml of bupivacaine heavy in all patients. After taking bone cuts & before the placement Corticosteroids of the implant, cocktail of the drug was infiltrated using sterile technique into 9 specific sites. -

Adverse Effects of Xenogenic Scaffolding in the Context of A
Lamas et al. Trials (2019) 20:387 https://doi.org/10.1186/s13063-019-3504-3 RESEARCH Open Access Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full- thickness rotator cuff tears José Ramón Lamas1†, Carlos García-Fernández2, Pilar Tornero-Esteban1, Yaiza Lópiz2, Luis Rodriguez-Rodriguez1, Luis Ortega3, Benjamín Fernández-Gutiérrez1*† and Fernando Marco2† Abstract Purpose: The purpose of the study was to compare the safety and efficacy of autologous mesenchymal stem cells (MSCs) embedded in a xenogenic scaffold for repairing the supraspinatus tendon. Methods: This was a randomized, double-blind and placebo-controlled trial evaluating patients with full-thickness rotator cuff tears (Eudra-CT, 2007–007630-19). Effectiveness was evaluated using the Constant score and a visual analogue pain scale (VAS). Constant score has four domains including pain (15 possible points), activities of daily living (20 possible points), mobility (40 possible points), and strength (25 possible points). Scores range from 0 points (most disability) to 100 points (least disability). The structural integrity of the repaired tendon was assessed by magnetic resonance imaging (MRI) according to Patte and Thomazeau classification criteria. The primary study end point was an improvement in the Constant score by 20 points at one year compared to initial assessment. Results: The trial was stopped due to adverse effects observed in both groups. Only thirteen patients were included and analyzed. The Constant questionnaire showed a significant improvement in the MSC treatment group compared with the preoperative data (p = 0.0073). Secondary outcome measures were similar in both groups. -

An Archetype Swing in Transdermal Drug Delivery
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 A COMPREHENSIVE REVIEW ON MICRONEEDLES - AN ARCHETYPE SWING IN TRANSDERMAL DRUG DELIVERY G. Ravi*, N. Vishal Gupta, M. P. Gowrav Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Shri Shivarathreeshwara Nagara, Mysuru, Karnataka, India. ARTICLE INFO ABSTRACT Article history Transdermal drug delivery is the non-invasive delivery of medications through the skin Received 23/12/2016 surface into the systemic circulation. The advantage of transdermal drug delivery system is Available online that it is painless technique of administration of drugs. The advantage of transdermal drug 31/01/2017 delivery system is that it is painless technique of administration of drugs. Transdermal drug delivery system can improve the therapeutic efficacy and safety of the drugs because drug Keywords delivered through the skin at a predetermined and controlled rate. Due to the various Microneedles, biomedical benefits, it has attracted many researches. The barrier nature of stratumcorneum Hypodermic Needles, poses a danger to the drug delivery. By using microneedles, a pathway into the human body Transdermal, can be recognized which allow transportation of macromolecular drugs such as insulin or Stratumcorneum, vaccine. These microneedles only penetrate outer layers of the skin, exterior sufficient not to Patch. reach the nerve receptors of the deeper skin. Thus the microneedles supplement is supposed painless and reduces the infection and injuries. Researches from the past few years showed that microneedles have emerged as a novel carrier and considered to be effective for safe and improved delivery of the different drugs. Microneedles development is created a new pathway in the drug delivery field. -

New Zealand Regulatory Guidelines for Medicines
New Zealand Regulatory Guidelines for Medicines Part G: Resources Edition 6.13 March 2011 (consolidation of fifth edition and subsequent updates) Table of Contents PART G: RESOURCES Section 1: Description of Dosage Form...............................................................................2 Section 2: Routes of Administration....................................................................................4 Section 3: Shelf Life and Storage Conditions .....................................................................5 Section 4: Abbreviations.......................................................................................................6 Description of Dosage Form The dosage form description for a product should be selected from the following list: Block Granules, oral Capsule Implant, subcutaneous Capsule, combination Implant, intracranial Capsule, liquid filled Implant, intraocular Capsule, modified release Infusion, concentrate Capsule, powder filled Infusion, emulsion Capsule, powder filled, nasal inhalation Infusion, powder for Capsule, soft gelatin Infusion, powder for concentrate Cement, bone, liquid component Infusion, solution Cement, bone, powder component Inhalation, capsule, liquid filled Cement, dental Inhalation, capsule, powder filled Chewing gum Inhalation, powder Chocolate, medicated Inhalation, solution Combination Inhalation, solution, powder for Condom, medicated Inhalation, suspension Condom with spermicide Inhalation, volatile liquid Cream, rectal Inhaler, aerosol, metered Cream, topical Injection -

JMSCR Vol||05||Issue||01||Page 15625-15629||January 2017
JMSCR Vol||05||Issue||01||Page 15625-15629||January 2017 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i1.71 Transdermal Patch: A Comprehensive Overview of Newer Drug Delivery System in Modern Medical Science Authors Dr Neelkanth Kote1, Dr. Poornima B2 Department of Physiology and Department of Pharmacology Raja Rajeswari Medical Collegeand Hospital Bangalore, Karnataka– 560074 Corresponding Author Dr Neelkanth Kote Department of Physiology, RRMC Bangalore, Karnataka -560074 Email: [email protected] Abstract Background: Oral route of drug administration has been the most common route of drug administration since the beginning of the therapeuticarea in medical science. However this route of drug administration fails at bypassing the first pass metabolism of the drug in liver with poor bio availability. Oral route of drug administration also fails in uncooperative /unconscious semiconscious / pediatric subjects. Transdermal patch, a new drug delivery system through skin to the parenteral circulation has been the most recently discussed topic in pharmacology and medicine as it is a noninvasive route of drug administration, delivered in a sustained / pre fixed dosage with no / extremely less first pass metabolism and better bio availability. Most importantly it can be used in semiconscious / unconscious & pediatric subjects without affecting their hemodynamics. The transdermal patches have many beneficial factors however they also come with some major drawbacks like varieties of formulation, design and quality of adhesiveness. This articles provides an insight of transdermal patch in terms of its mechanism of action, advantages, disadvantages and future scope of it in the medical science. -

Transdermal Nicotine Maintenance Attenuates the Subjective And
Neuropsychopharmacology (2004) 29, 991–1003 & 2004 Nature Publishing Group All rights reserved 0893-133X/04 $25.00 www.neuropsychopharmacology.org Transdermal Nicotine Maintenance Attenuates the Subjective and Reinforcing Effects of Intravenous Nicotine, but not Cocaine or Caffeine, in Cigarette-Smoking Stimulant Abusers 1 1 ,1,2 Bai-Fang X Sobel , Stacey C Sigmon and Roland R Griffiths* 1Department of Psychiatry and Behavioral Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA The effects of transdermal nicotine maintenance on the subjective, reinforcing, and cardiovascular effects of intravenously administered cocaine, caffeine, and nicotine were examined using double-blind procedures in nine volunteers with histories of using tobacco, caffeine, and cocaine. Each participant was exposed to two chronic drug maintenance phases (21 mg/day nicotine transdermal patch and placebo transdermal patch). Within each drug phase, the participant received intravenous injections of placebo, cocaine (15 and 30 mg/70 kg), caffeine (200 and 400 mg/70 kg), and nicotine (1.0 and 2.0 mg/70 kg) in mixed order across days. Subjective and cardiovascular data were collected before and repeatedly after drug or placebo injection. Reinforcing effects were also assessed after each injection with a Drug vs Money Multiple-Choice Form. Intravenous cocaine produced robust dose-related increases in subjective and reinforcing effects; these effects were not altered by nicotine maintenance. Intravenous caffeine produced elevations on several subjective ratings; nicotine maintenance did not affect these ratings. Under the placebo maintenance condition, intravenous nicotine produced robust dose-related subjective effects, with maximal increases similar to the high dose of cocaine; nicotine maintenance significantly decreased the subjective and reinforcing effects of intravenous nicotine. -

A Brief Review on Transdermal Patches
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 7 Issue 2 - June 2018 Copyright © All rights are reserved by Nidhi Sharma DOI: 10.19080/OMCIJ.2018.07.555707 A Brief Review on Transdermal Patches Nidhi Sharma* HIMT College of Pharmacy, Greater Noida, India Submission: May 12, 2018; Published: June 05, 2018 *Corresponding author: Nidhi Sharma, HIMT College of Pharmacy, Greater Noida, India, Email: Abstract healingTransdermal to an injured drug area delivery of the system body. was An presentedadvantage toof overcome a transdermal the difficulties drug delivery of drug route delivery over especiallyother types oral of route. delivery A transdermal system such patch as oral, is a topical,medicated i.v., adhesive i.m., etc. ispatch that that the patchis placed provides on the askin controlled to deliver release a specific of the dose medication of medication into the through patient, the usually skin and through into the either bloodstream. a porous It membrane promotes covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier, as a result, only medications whose molecules are small can easily penetrate the skin, so it can be delivered by this method. This review article describes the overall introduction of transdermal patches including type of transdermal patches, method of preparation of transdermal -
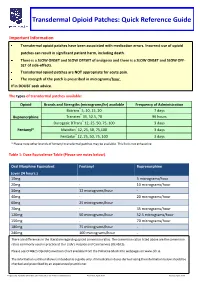
Transdermal Opioid Patches: Quick Reference Guide
Transdermal Opioid Patches: Quick Reference Guide Important Information Transdermal opioid patches have been associated with medication errors. Incorrect use of opioid patches can result in significant patient harm, including death. There is a SLOW ONSET and SLOW OFFSET of analgesia and there is a SLOW ONSET and SLOW OFF- SET of side-effects. Transdermal opioid patches are NOT appropriate for acute pain. The strength of the patch is prescribed in micrograms/hour. If in DOUBT seek advice. The types of transdermal patches available: Opioid Brands and Strengths (micrograms/hr) available Frequency of Administration Butrans® 5, 10, 15, 20 7 days Buprenorphine Transtec® 35, 52.5, 70 96 hours Durogesic DTrans® 12, 25, 50, 75, 100 3 days Fentanyl* Matrifen® 12, 25, 50, 75,100 3 days Fentadur® 12, 25, 50, 75, 100 3 days * Please note other brands of fentanyl transdermal patches may be available. This list is not exhaustive. Table 1: Dose Equivalence Table (Please see notes below). Oral Morphine Equivalent Fentanyl Buprenorphine (over 24 hours.) 10mg 5 micrograms/hour 20mg - 10 micrograms/hour 30mg 12 micrograms/hour - 40mg - 20 micrograms/hour 60mg 25 micrograms/hour 70mg - 35 micrograms/hour 120mg 50 micrograms/hour 52.5 micrograms/hour 150mg - 70 micrograms/hour 180mg 75 micrograms/hour - 240mg 100 micrograms/hour - There are differences in the literature regarding opioid conversion ratios. The conversion ratios listed above are the conversion ratios commonly used in practice at Our Lady’s Hospice and Care Service (OLH&CS). Please see OLH&CS Opioid Conversion Chart available from the Palliative Meds Info webpages on www.olh.ie. -
Aptensio XR (Methylphenidate) Extended-Release Capsules
Aptensio XR™ (methylphenidate) – First-time authorized brand alternative • On May 5, 2020, Rhodes Pharmaceuticals launched an authorized brand alternative of its (Rhodes) branded Aptensio XR (methylphenidate) extended-release capsules. • Aptensio XR is approved for the treatment of attention deficit hyperactivity disorder (ADHD) in patients 6 years and older. • Methylphenidate is also available generically as an oral solution, extended-release capsule, extended-release tablet, tablet, and chewable tablet. It is also available as a brand extended-release capsule (Adhansia XR™, Jornay PM™), extended-release orally disintegrating tablet (Contempla-XR ODT™), transdermal patch (Daytrana®), extended-release chewable tablet (QuilliChew XR®), extended-release oral suspension (Quillivant XR®), and extended-release tablet (Relexxii™). — Methylphenidate oral solution, tablet, chewable tablet and extended-release tablets are approved to treat ADHD and narcolepsy. — Methylphenidate extended-release capsules, Adhansia XR, Contempla-XR ODT, Daytrana, Jornay PM, QuilliChew XR, Quillivant XR and Relexxii are approved to treat ADHD. • Aptensio XR carries a boxed warning for abuse and dependence. optumrx.com OptumRx® specializes in the delivery, clinical management and affordability of prescription medications and consumer health products. We are an Optum® company — a leading provider of integrated health services. Learn more at optum.com. All Optum® trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their respective owners. This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written consent of OptumRx. RxNews® is published by the OptumRx Clinical Services Department. ©2020 Optum, Inc. All rights reserved. . -

Transdermal and Transbuccal Drug Delivery
TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS by LONSHENG HU A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Pharmaceutical Science written under the direction of Bozena Michniak-Kohn and approved by ________________________ ________________________ ________________________ ________________________ New Brunswick, New Jersey October, 2010 ABSTRACT OF THE DISSERTATION TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS By LONSHENG HU Dissertation Director: Professor Bozena Michniak-Kohn Transdermal and transbuccal routes offer attractive alternatives for systemic delivery of drugs due to their distinct advantages: non-invasive, avoidance of first-pass effect, improved bioavailability and reduction of systemic side effects. However, only a few drugs have been successfully delivered into blood stream to reach therapeutic levels without causing notable skin irritation or damage. Transbuccal drug delivery systems are still at research stage. The major barriers to transdermal and transbuccal drug delivery are stratum corneum of skin and epithelium of buccal tissue. The objective of this work was to overcome these barriers to significantly enhance transdermal and transbuccal delivery of hydrophilic drugs without causing major damage to skin and buccal tissue. In this work, iontophoresis, chemical