The Gas Laws A. Introduction. 1. Comparison of Three States of Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -
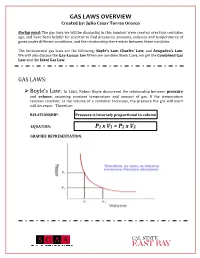
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant. -

Chapter 14: Gases
418-451_Ch14-866418 5/9/06 6:19 PM Page 418 CHAPTER 14 Gases Chemistry 2.a, 3.c, 3.d, 3.e, 4.a, 4.c, 4.d, 4.e, 4.f, 4.g, 4.h I&E 1.b, 1.c, 1.d What You’ll Learn ▲ You will use gas laws to cal- culate how pressure, tem- perature, volume, and number of moles of a gas will change when one or more of these variables is altered. ▲ You will compare properties of real and ideal gases. ▲ You will apply the gas laws and Avogadro’s principle to chemical equations. Why It’s Important From barbecuing on a gas grill to taking a ride in a hot-air balloon, many activities involve gases. It is important to be able to predict what effect changes in pressure, temperature, volume, or amount, will have on the properties and behavior of a gas. Visit the Glencoe Chemistry Web site at chemistrymc.com to find links about gases. Firefighters breathe air that has been compressed into tanks that they can wear on their backs. 418 Chapter 14 418-451_Ch14-866418 5/9/06 6:19 PM Page 419 DISCOVERY LAB More Than Just Hot Air Chemistry 4.a, 4.c I&E 1.d ow does a temperature change affect the air in Ha balloon? Safety Precautions Always wear goggles to protect eyes from broken balloons. Procedure 1. Inflate a round balloon and tie it closed. 2. Fill the bucket about half full of cold water and add ice. 3. Use a string to measure the circumference of the balloon. -

The Ideal and Combined Gas Laws PV = Nrt Or P1V1 = P2V2 T1 T2
The Ideal and Combined Gas Laws PV = nRT or P1V1 = P2V2 T1 T2 Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be PV = nRT 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? 2) If I initially have a gas with a pressure of 84 kPa and a temperature of 350 C and I heat it an additional 230 degrees, what will the new pressure be? Assume the volume of the container is constant. 3) My car has an internal volume of 2600 liters. If the sun heats my car from a temperature of 200 C to a temperature of 550 C, what will the pressure inside my car be? Assume the pressure was initially 760 mm Hg. 4) How many moles of gas are in my car in problem #3? 5) A toy balloon filled with air has an internal pressure of 1.25 atm and a volume of 2.50 L. If I take the balloon to the bottom of the ocean where the pressure is 95 atmospheres, what will the new volume of the balloon be? How many moles of gas does the balloon hold? (Assume T = 285 K) For chemistry help, visit www.chemfiesta.com © 2000 Cavalcade Publishing - All Rights Reserved MIXED GAS LAWS WORKSHEET Created by Tara L. Moore at www.learning.mgccc.cc.ms.us/pk/sciencedocs/gaslawwksheet.htm Directions: Answer each question below. -

The Effects of Carbonated Beverages on Arterial Oxygen Saturation, Serum Hemoglobin Concentration and Maximal Oxygen Consumption
AN ABSTRACT OF THE THESIS OF Max Waibler for the degree of Master of Science in Human Performance presented on August 21. 1991. Title : The Effects of Carbonated Beverages on Arterial Oxygen Saturation.Serum Hemoglobin Concentration and Maximal Oxygen Consumption Abstract approved :_Redacted for Privacy Dr. AWthony R. Wilcox Elite milers, Sir Roger Bannister and Joseph Falcon, have stated that the consumption of carbonated beverages hinders the performance of aerobic events. Oxygen transport is purportedly impaired by the consumption of carbonated beverages. The research on carbonated beverages has been limited to the effects on the digestive system, gastric emptying, and thermal heat stress in animals. The purpose of this study was to investigate the effects of consuming 28 ounces of carbonated beverages per day, for three weeks,on arterial oxygen saturation (Sa02), serum hemoglobin concentrations (Hb), and maximal oxygen consumption (VO2max) in experienced cyclists. Nine competitive cyclists and triathletes (aged 19-24 years, M= 21.67 years), with average weights and percent body fat of 76.51 kg and 11.4 percent respectively, were randomly assigned to a three week period of consuming 28 ounces of carbonated water or a three week period of no carbonated beverages. At the end of each three week period, a 5 c.c. blood sample was taken for Hb determination and the subjects performed a test of maximal oxygen consumption on a cycle ergometer while Sa02 was being monitored. The groups then crossed-over with respect to their treatment, and after another three week period, the same variables were measured. The Student's tstatistic was used to compare Sa02, Hb, and VO2max. -

Empirical Gas Laws
Empirical Gas Laws • Early experiments conducted to understand the relationship between P, V and T (and number of moles n) • Results were based purely on observation – No theoretical understanding of what was taking place • Systematic variation of one variable while measuring another, keeping the remaining variables fixed Boyle’s Law (1662) • For a closed system (i.e. no gas can enter or leave) undergoing an isothermal process (constant T), there is an inverse relationship between the pressure and volume of a gas (regardless of the identity of the gas) V α 1/P • To turn this into an equality, introduce a constant of proportionality (a) à V = a/P, or PV = a • Since the product of PV is equal to a constant, it must UNIVERSALLY (for all values of P and V) be equal to the same constant P1V1 = P2V2 Boyle’s Law • There is an inverse relationship between P and V (isothermal, closed system) https://en.wikipedia.org/wiki/Boyle%27s_law Charles’ Law (1787) • For a closed system undergoing an isobaric process (constant P), there is a direct relationship between the volume and temperature of a gas. V α T • Proceeding in a similar fashion as before, we can say that V = bT (the constant need not the same as that of Boyle’s Law!) and therefore V/T = b Charles’ Law (continued) • This equation presumes a plot of V vs T would give a straight line with a slope of b and a y-intercept of 0 (i.e. equation goes through the origin). • However the experimental data did have an intercept! • Lord Kelvin (1848) decided to extrapolate the data to see where it would -

Introduction Boyle's
BASIC GAS LAWS - 2012 NGA GAS OPERATIONS SCHOOL INTRODUCTION There are a number of very important reasons why everyone connected to the natural gas industry should understand the Basic Gas Laws. (1) Designing, building, and maintaining safe gas distribution and transmission systems can only be done by understanding and applying the laws of physics governing the behavior of natural gas. (2) Effective job performance in the natural gas industry requires an understanding of the technical language we encounter every day, a substantial portion of which is directly and indirectly related to the behavior of natural gas. (3) Safety is perhaps the most important driving force in our industry and many of the safety issues employees face relate to understanding how gases behave. (4) Many of the service issues arising from interaction with gas customers involve questions of measurement accuracy, pressure, and terminology that are not at all understood by end users but are related to the application of Basic Gas Laws. This class is not intended to cover the subject matter in depth but instead will focus on (3) Basic Gas Laws that everyone should conceptually understand. BOYLE'S LA W - (PRESSUREJ Of the (3) Basic Gas Laws discussed in this presentation, Boyle's Law describing the effect of pressure on gas is the most commonly applied and far-reaching in its impact in our industry. Therefore it will get the most attention in this presentation. For those who are not engineers or mathematically inclined, a very simple example of Boyle's Law at work in the real world is the use of scuba tanks by divers that need to work (and breathe) underwater. -

Atmospheric Thermodynamics
Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth’s atmosphere? Gaseous Constituents of the Earth’s atmosphere (dry air) Fractional Concentration by Constituent Molecular Weight Volume of Dry Air Nitrogen (N2) 28.013 78.08% Oxygen (O2) 32.000 20.95% Argon (Ar) 39.95 0.93% Carbon Dioxide (CO2) 44.01 380 ppm Neon (Ne) 20.18 18 ppm Helium (He) 4.00 5 ppm Methane (CH4) 16.04 1.75 ppm Krypton (Kr) 83.80 1 ppm Hydrogen (H2) 2.02 0.5 ppm Nitrous oxide (N2O) 44.013 0.3 ppm Ozone (O3) 48.00 0-0.1 ppm Water vapor is present in the atmosphere in varying concentrations from 0 to 5%. Aerosols – solid and liquid material suspended in the air What are some examples of aerosols? The particles that make up clouds (ice crystals, rain drops, etc.) are also considered aerosols, but are more typically referred to as hydrometeors. We will consider the atmosphere to be a mixture of two ideal gases, dry air and water vapor, called moist air. Gas Laws Equation of state – an equation that relates properties of state (pressure, volume, and temperature) to one another Ideal gas equation – the equation of state for gases pV = mRT p – pressure (Pa) V – volume (m3) € m – mass (kg) R – gas constant (value depends on gas) (J kg-1 K-1) T – absolute temperature (K) This can be rewritten as: m p = RT V p = ρRT r - density (kg m-3) € or as: V p = RT m pα = RT a - specific volume (volume occupied by 1 kg of gas) (m3 kg-1) € Boyle’s Law – for a fixed mass of gas at constant temperature V ∝1 p Charles’ Laws: For a fixed mass of gas at constant pressure V ∝T € For a fixed mass of gas at constant volume p ∝T € € Mole (mol) – gram-molecular weight of a substance The mass of 1 mol of a substance is equal to the molecular weight of the substance in grams. -

133 Chapter 8: Gases and Gas Laws. the First Substances to Be Produced
133 Chapter 8: Gases and Gas Laws. The first substances to be produced and studied in high purity were gases. Gases are more difficult to handle and manipulate than solids and liquids, since any minor mistakes generally results in the gas escaping to the atmosphere. However, the ability to produce gases in very high purity made the additional difficulty acceptable. The most common way of producing a gas was by some sort of chemical reaction, and the gas was collected by liquid displacement (either water or mercury). Figure 8.1 shows the general process of collecting gas by liquid displacement. Figure 8.1. Method of displacement for collecting gases. A water-filled container is inverted and placed into a water trough. A rubber hose is placed in the mouth of the container, with the other end attached to a reaction flask. The chemical reaction produces gas, which flows through the tube and displaces water from the container. By selecting the proper reactant masses, sufficient gas to fill 3 – 6 containers can be produced. Typically, the first container collected isn’t saved, since it contains residual air from the reaction flask. Using this general method, scientists produced and characterized hydrogen, oxygen, nitrogen, carbon dioxide, sulfur dioxide, chlorine, and several other gases. Once they obtained reasonably pure gases, systematic experimentation led to other discoveries. Generally, gases have properties substantially different than solids or liquids. Gases do not have fixed volumes; instead their volume depends directly upon pressure and temperature. Gases don’t have a fixed shape, but are said to “take the shape of their container”. -

Respiratory Physiology
Disclaimer: The Great Ormond Street Paediatric Intensive Care Training Programme was developed in 2004 by the clinicians of that Institution, primarily for use within Great Ormond Street Hospital and the Children’s Acute Transport Service (CATS). The written information (known as Modules) only forms a part of the training programme.The modules are provided for teaching purposes only and are not designed to be any form of standard reference or textbook. The views expressed in the modules do not necessarily represent the views of all the clinicians at Great Ormond Street Hospital and CATS. The authors have made considerable efforts to ensure the information contained in the modules is accurate and up to date. The modules are updated annually. Users of these modules are strongly recommended to confirm that the information contained within them, especially drug doses, is correct by way of independent sources.The authors accept no responsibility for any inaccuracies, information perceived as misleading, or the success of any treatment regimen detailed in the modules. The text, pictures, images, graphics and other items provided in the modules are copyrighted by “Great Ormond Street Hospital” or as appropriate, by the other owners of the items. Copyright 2004-2005 Great Ormond Street Hospital. All rights reserved. YEAR 1 ITU CURRICULUM 4 - Respiratory Physiology Andy Petros, September 2005 Updated Catherine Sheehan, Sanjiv Sharma, July 2012 Associated clinical guidelines/protocols: Fundamental Knowledge: List of topics relevant to PIC that will have been covered in membership examinations. They will not be repeated here. Anatomy: Anatomy of upper and lower respiratory tract, mediastinum, rib cage, muscles of respiration and diaphragm. -

M.Sc. in Meteorology
M.Sc. in Meteorology Physical Meteorology Prof Peter Lynch Mathematical Computation Laboratory Dept. of Maths. Physics, UCD, Belfield. Part 2 Atmospheric Thermodynamics 2 Atmospheric Thermodynamics Thermodynamics plays an important role in our quanti- tative understanding of atmospheric phenomena, ranging from the smallest cloud microphysical processes to the gen- eral circulation of the atmosphere. The purpose of this section of the course is to introduce some fundamental ideas and relationships in thermodynam- ics and to apply them to a number of simple, but important, atmospheric situations. The course is based closely on the text of Wallace & Hobbs 3 Outline of Material 4 Outline of Material • 1 The Gas Laws 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation • 3 The First Law of Thermodynamics 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation • 3 The First Law of Thermodynamics • 4 Adiabatic Processes 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation • 3 The First Law of Thermodynamics • 4 Adiabatic Processes • 5 Water Vapour in Air 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation • 3 The First Law of Thermodynamics • 4 Adiabatic Processes • 5 Water Vapour in Air • 6 Static Stability 4 Outline of Material • 1 The Gas Laws • 2 The Hydrostatic Equation • 3 The First Law of Thermodynamics • 4 Adiabatic Processes • 5 Water Vapour in Air • 6 Static Stability • 7 The Second Law of Thermodynamics 4 The Kinetic Theory of Gases The atmosphere is a gaseous envelope surrounding the Earth. -
Standards and Procedures
Standards and Procedures Version 5.0a March 2020 UTD Scuba Diving, LLC 4653 Carmel Mountain Road, Suite 308-301 San Diego, CA, 92130 USA [email protected] www.utdscubadiving.com +1 760-654-5533 © 2020 UTD Scuba Diving, LLC v5.0a All Rights Reserved Table of Contents 1.0 General ...................................................................................................................................7 1.1 Overview .............................................................................................................................7 1.1.1 Our Mission ......................................................................................................................7 1.1.2 Unified Team Diving (UTD) Ethos ....................................................................................7 1.1.3 The UTD Covenants .........................................................................................................8 1.1.4 The UTD Certification Philosophy ....................................................................................8 1.1.5 Overall UTD Teaching Philosophies .................................................................................9 1.2.1 Training Steps ..................................................................................................................9 1.3 Training Process and Definitions .........................................................................................9 1.3.1 Overall Process ................................................................................................................9