The Mathematics Behind Partial Pressure Gas Blending Or "Why You Shouldn't Calculate Your Mix in Your Head"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Escherichia Coli HD701
The Process Intensification of Biological Hydrogen Production by Escherichia coli HD701 By Michael Sulu A thesis submitted to The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Chemical Engineering College of Engineering and Physical Sciences The University of Birmingham November 2009 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract Hydrogen is seen as a potential fuel for the future; its choice is driven by the increasing awareness of the necessity for clean fuel. Together with the simultaneous development of “green technologies” and sustainable development, a current goal is to convert waste to energy or to create energy from a renewable resource. Biological processing [of renewables] or bioremediation of waste to create hydrogen as a product fulfils this goal and, as such, is widely researched. In this work, an already established process, using a hydrogenase up‐regulated strain ‐ was characterised and the important process parameters were established. This bacterial strain has the potential for industrial‐scale hydrogen production from, for example, waste sugars. Previous work, repeated here, showed that hydrogen could be generated by E. -

Professional and Honor Program - Descriptions with Prerequisite Requirements
Professional and Honor Program - Descriptions with Prerequisite Requirements Professional Career Programs - Full Residence Honor Programs The Professional Career Programs provide broad training for students seeking Short Residence with Self Study Prep rewarding, full-time employment in the recreational diving industry. Both The Institute's Honor programs are shortened versions of our full residence full-residence individual skill and combination skill programs are available. “Professional” Instructor, Divemaster /Dive Control Specialist and Rescue Areas covered in professional programs are: skin and scuba diving instruc- training modules. We designed the “Honor” Programs to give credit for tion, divemaster supervision and dive control specialist, boatmaster, under- certain types of previous training from diver to divemaster and for using our water digital photography and videography with computer editing, promo- self study package to help prepare before attendance. tional diving video-DVD-CD and photo production, detailed dive business, store and resort operations, diving business sales and persuasion, diving Honor program entry requirements are a lot stiffer than our longer full equipment overhaul and repair technology, deep and technical diving residence “Professional” programs. They also do not include the broad instruction, semi-closed or closed circuit rebreather instruction, diving variety of training and practice available in our more extensive "Professional" specialties instruction, submersibles and underwater communication tech- programs. nology, air station and technical gas blending operations, professional CPR- People attending shorter Honor Programs, need to be self-motivated with First Aid, and diving accident response technology and instruction. excellent reading and math skills. Most Honor Programs have physical and Making a good living in the recreational diving industry generally requires written attendance qualification exams to test your preparedness before you more abilities than just teaching. -

A Gas Dilution System for Arsine-Air Mixtures
NBSIR 73-260 A Gas Dilution System for Arsine-Air Mixtures Peter A. Pella, Ernest E. Hughes, and John K. Taylor National Bureau of Standards Department of Commerce Washington, D. C. 20234 October 1973 Final Report Prepared for National Institute for Occupational Safety and Health Division of Laboratories and Criteria Development Cincinnati, Ohio 45202 NBSIR 73-260 A GAS DILUTION SYSTEM FOR ARSINE-AIR MIXTURES Peter A. Pella, Ernest E. Hughes, and John K. Taylor National Bureau of Standards Department of Commerce Washington, D. C. 20234 October 1973 Final Report Prepared for National Institute for Occupational Safety and Health Division of Laboratories and Criteria Development Cincinnati, Ohio 45202 I U. S. DEPARTMENT OF COMMERCE, Frederick B. Dent, Secretary NATIONAL BUREAU OF STANDARDS, Richard W. Roberts, Director A Gas Dilution System for Arsine-Air Mixtures ABSTRACT A gas -blending system originally designed for chlorine-air mixtures was modified for producing arsine-air mixtures in the concentration range from 0.02 to 0.25 ppm. This system has been tested in order to provide accurately known concentrations of arsine- in-air for calibration of analytical monitor- ing devices. An analytical method has been developed for checking the concentration of arsine in the work- ing standard and consists of the spectrophotometric measurement of an arsine-diethyldithiocarbamate complex in solution. 1. INTRODUCTION The system originally designed for preparing chlorine- air mixtures [1] has been adapted for producing arsine-air mixtures in the range from 0.02 to 0.25 ppm. The system combines a gas blending unit and an analytical unit. The gas blending unit produces concentrations of arsine by dilu- tion of a relatively high concentration of arsine in nitrogen (i.e. -

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -

15. Advanced Gas Blender
TDI Standards and Procedures Part 2: TDI Diver Standards 15. Advanced Gas Blender 15.1 Introduction This course enables the successful candidate to engage in the blending of oxygen and helium based gasses. The objective of this course is to train candidates in the proper procedures needed for the preparation and blending of high quality nitrox and trimix gases for use in technical diving. 15.2 Qualifications of Graduates Upon successful completion of this course candidates will be able to prepare high quality scuba gases. 15.3 Who May Teach Any active TDI Advanced Gas Blending Instructor may teach this course 15.4 Student to Instructor Ratio Academic 1. Unlimited, so long as adequate facility, supplies and time are provided to ensure comprehensive and complete training of subject matter Confined Water (swimming pool-like conditions) 1. N/A Open Water (ocean, lake, quarry, spring, river or estuary) 1. N/A 15.5 Student Prerequisites 1. Minimum age 18 2. Provide proof of certification as a TDI Nitrox Gas Blender or equivalent Version 0221 113 TDI Standards and Procedures Part 2: TDI Diver Standards 15.6 Course Structure and Duration Open Water Execution 1. N/A Course Structure 1. TDI allows instructors to structure courses according to the number of students participating and their skill level Duration 1. The minimum number of classroom and briefing hours is 6 15.7 Administrative Requirements The following are the administrative tasks: 1. Collect the course fees from all the students 2. Ensure that the students have the required equipment 3. Communicate the training schedule to the students 4. -

Diving Air Compressor - Wikipedia, the Free Encyclopedia Diving Air Compressor from Wikipedia, the Free Encyclopedia
2/8/2014 Diving air compressor - Wikipedia, the free encyclopedia Diving air compressor From Wikipedia, the free encyclopedia A diving air compressor is a gas compressor that can provide breathing air directly to a surface-supplied diver, or fill diving cylinders with high-pressure air pure enough to be used as a breathing gas. A low pressure diving air compressor usually has a delivery pressure of up to 30 bar, which is regulated to suit the depth of the dive. A high pressure diving compressor has a delivery pressure which is usually over 150 bar, and is commonly between 200 and 300 bar. The pressure is limited by an overpressure valve which may be adjustable. A small stationary high pressure diving air compressor installation Contents 1 Machinery 2 Air purity 3 Pressure 4 Filling heat 5 The bank 6 Gas blending 7 References 8 External links A small scuba filling and blending station supplied by a compressor and Machinery storage bank Diving compressors are generally three- or four-stage-reciprocating air compressors that are lubricated with a high-grade mineral or synthetic compressor oil free of toxic additives (a few use ceramic-lined cylinders with O-rings, not piston rings, requiring no lubrication). Oil-lubricated compressors must only use lubricants specified by the compressor's manufacturer. Special filters are used to clean the air of any residual oil and water(see "Air purity"). Smaller compressors are often splash lubricated - the oil is splashed around in the crankcase by the impact of the crankshaft and connecting A low pressure breathing air rods - but larger compressors are likely to have a pressurized lubrication compressor used for surface supplied using an oil pump which supplies the oil to critical areas through pipes diving at the surface control point and passages in the castings. -

General Training Standards, Policies, and Procedures
General Training Standards, Policies, and Procedures Version 9.2 GUE General Training Standards, Policies, and Procedures © 2021 Global Underwater Explorers This document is the property of Global Underwater Explorers. All rights reserved. Unauthorized use or reproduction in any form is prohibited. The information in this document is distributed on an “As Is” basis without warranty. While every precaution has been taken in its preparation, neither the author(s) nor Global Underwater Explorers have any liability to any person or entity with respect to any loss or damage caused or alleged to be caused, directly or indirectly, by this document’s contents. To report violations, comments, or feedback, contact [email protected]. 2 GUE General Training Standards, Policies, and Procedures Version 9.2 Contents 1. Purpose of GUE .............................................................................................................................................6 1.1 GUE Objectives ............................................................................................................................................. 6 1.1.1 Promote Quality Education .................................................................................................................. 6 1.1.2 Promote Global Conservation Initiatives .......................................................................................... 6 1.1.3 Promote Global Exploration Initiatives ............................................................................................. 6 -

Case Study: Membrane Co2 Removal from Natural Gas, Grissik Gas Plant, Sumatra, Indonesia
CASE STUDY: MEMBRANE CO2 REMOVAL FROM NATURAL GAS, GRISSIK GAS PLANT, SUMATRA, INDONESIA Charles L. Anderson Air Liquide – MEDAL, L.P. Newport, Delaware 19804 USA (1) 302 225-2102 [email protected] Anggiat Siahaan ConocoPhillips Jakarta, Indonesia (62) (21) 524-1987 [email protected] Abstract One of the world’s largest membrane systems is used for bulk removal of CO2 from natural gas at the Grissik gas processing plant in South Sumatra, Indonesia. The membrane system processes 310 MMSCFD of natural gas, reducing CO2 from 30% to 15%. The Grissik plant is termed a membrane plus amine hybrid which offers particularly attractive operational benefits. The membrane system has been operating for more than four years with no membrane replacement and offers the benefit of very long membrane life. CASE STUDY: MEMBRANE CO2 REMOVAL FROM NATURAL GAS, GRISSIK GAS PLANT, SUMATRA, INDONESIA Charles L. Anderson, Air Liquide – MEDAL, L.P., Newport, Delaware, USA Anggiat Siahaan, ConocoPhillips, Jakarta, Indonesia Introduction ConocoPhillips operates the Grissik gas processing plant on behalf of its partners, Talisman Energy Inc., Pertamina and BPMigas. The Grissik plant, located in South Sumatra, Indonesia, pictured in Figure 1, processes 310 MMSCFD of natural gas, primarily reducing CO2 concentration from 30% in the raw feed down to 3% in the sales gas. Figure 1. Grissik Gas Plant Process Overview The Grissik plant CO2 removal process utilizes both membrane separation and amine absorption technology, termed a membrane plus amine hybrid. A simplified process flow diagram is shown in Figure 2. The Thermal Swing Adsorption (TSA) removes heavy hydrocarbons, serving the triple function of membrane pre-treatment, feed gas dehydration and sales gas hydrocarbon dew pointing. -

TDI Advanced Nitrox Diver
TDI Advanced Nitrox Diver Are you looking to expand your dive time? Maybe you’re a scientific diver or photographer looking to stay in the water a little longer?The TDI Advanced Nitrox Course qualifies divers to use enriched air nitrox from EAN 21 through EAN 100 percent within your current certification level to a maximum depth of 40 metres/130 feet during dives that do not require staged decompression. Often taught in conjunction with the TDI Decompression Procedures course, this can be considered the foundation of your technical diving career. TDI Advanced Nitrox is also a must for SCR or CCR divers. Who this course is for: • The certified nitrox diver looking to expand their understanding of nitrox mixtures containing more than 40% oxygen • The certified nitrox diver looking to expand their in-water skills • The certified diver who has interest in moving forward with technical diving education Course prerequisites (these requirements must be met prior to the start of the course): • Minimum age 18, 15 with parental consent • Minimum certification of TDI Nitrox Diver or equivalent • Proof of 25 logged open water dives What you can expect to learn: Advanced Nitrox picks up where TDI Nitrox leaves off and offers a more in-depth look at diving with nitrox including: • Physics and physiology relating to diving with gas mixes containing more than 40% oxygen • Gas planning, dive tables, dive computers, oxygen limitations, nitrogen limitations • Equipment considerations, cylinder labeling, analyzing nitrox mixtures, gas blending procedures, -
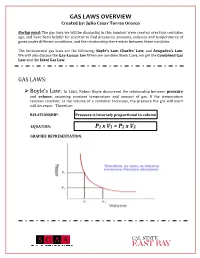
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant. -

The Gas Laws A. Introduction. 1. Comparison of Three States of Matter
CHAPTER FIVE: THE GASEOUS STATE Part One: The Gas Laws A. Introduction. 1. Comparison of three states of matter: solids condensed states liquids (high density, hard to compress) fluids (flow freely) gases (low density, easy to compress) 1 mole liquid H2O occupies 18 mL 1 mole H2O vapor at 100° C and atmospheric pressure occupies 30,600mL Thus, gas molecules must be far apart compared to molecular sizes and interact only weakly. 2. Composition of dry air by volume: 78% N2, 21% O2, 1% Ar, traces of other. 3. Properties of gases: a. Easily compressed into small volumes by applying pressure. b. Exert a pressure P on their surroundings; an equal pressure must be applied to confine them. c. Expand without limit to uniformly and completely occupy the volume of any container. d. Individual molecules exhibit a chaotic motion called diffusion. e. Properties described by gas laws. Show them “A Little Box of Air.” Chapter 5 Page 1 B. Pressure (P). (Section 5.1) 1. P = force per unit area produced by incessant collisions of particles with container walls. 2. Measurement of atmospheric pressure (Torricelli barometer): h ∝ pressure average height h: = 760 mm Hg at sea level P = 760 mm Hg = 1 atmosphere (atm) ≈ 30 inches 1 mm Hg = 1 “torr” SI unit of P is the pascal (Pa) 760 mm Hg: = 1 atm = 1.01325 x 105 Pa = 101.325kPa 3. Pressure of a column of liquid = hydrostatic pressure: P = gdh = accel. of gravity x density of liquid x height of column 2 g=9.81 m/s Chapter 5 Page 2 4. -

Chapter 14: Gases
418-451_Ch14-866418 5/9/06 6:19 PM Page 418 CHAPTER 14 Gases Chemistry 2.a, 3.c, 3.d, 3.e, 4.a, 4.c, 4.d, 4.e, 4.f, 4.g, 4.h I&E 1.b, 1.c, 1.d What You’ll Learn ▲ You will use gas laws to cal- culate how pressure, tem- perature, volume, and number of moles of a gas will change when one or more of these variables is altered. ▲ You will compare properties of real and ideal gases. ▲ You will apply the gas laws and Avogadro’s principle to chemical equations. Why It’s Important From barbecuing on a gas grill to taking a ride in a hot-air balloon, many activities involve gases. It is important to be able to predict what effect changes in pressure, temperature, volume, or amount, will have on the properties and behavior of a gas. Visit the Glencoe Chemistry Web site at chemistrymc.com to find links about gases. Firefighters breathe air that has been compressed into tanks that they can wear on their backs. 418 Chapter 14 418-451_Ch14-866418 5/9/06 6:19 PM Page 419 DISCOVERY LAB More Than Just Hot Air Chemistry 4.a, 4.c I&E 1.d ow does a temperature change affect the air in Ha balloon? Safety Precautions Always wear goggles to protect eyes from broken balloons. Procedure 1. Inflate a round balloon and tie it closed. 2. Fill the bucket about half full of cold water and add ice. 3. Use a string to measure the circumference of the balloon.