Presented by the NOAA Diving Center Seattle, Washington Global View
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Page 1 of 6 This Is Henry's Law. It Says That at Equilibrium the Ratio of Dissolved NH3 to the Partial Pressure of NH3 Gas In
CHMY 361 HANDOUT#6 October 28, 2012 HOMEWORK #4 Key Was due Friday, Oct. 26 1. Using only data from Table A5, what is the boiling point of water deep in a mine that is so far below sea level that the atmospheric pressure is 1.17 atm? 0 ΔH vap = +44.02 kJ/mol H20(l) --> H2O(g) Q= PH2O /XH2O = K, at ⎛ P2 ⎞ ⎛ K 2 ⎞ ΔH vap ⎛ 1 1 ⎞ ln⎜ ⎟ = ln⎜ ⎟ − ⎜ − ⎟ equilibrium, i.e., the Vapor Pressure ⎝ P1 ⎠ ⎝ K1 ⎠ R ⎝ T2 T1 ⎠ for the pure liquid. ⎛1.17 ⎞ 44,020 ⎛ 1 1 ⎞ ln⎜ ⎟ = − ⎜ − ⎟ = 1 8.3145 ⎜ T 373 ⎟ ⎝ ⎠ ⎝ 2 ⎠ ⎡1.17⎤ − 8.3145ln 1 ⎢ 1 ⎥ 1 = ⎣ ⎦ + = .002651 T2 44,020 373 T2 = 377 2. From table A5, calculate the Henry’s Law constant (i.e., equilibrium constant) for dissolving of NH3(g) in water at 298 K and 340 K. It should have units of Matm-1;What would it be in atm per mole fraction, as in Table 5.1 at 298 K? o For NH3(g) ----> NH3(aq) ΔG = -26.5 - (-16.45) = -10.05 kJ/mol ΔG0 − [NH (aq)] K = e RT = 0.0173 = 3 This is Henry’s Law. It says that at equilibrium the ratio of dissolved P NH3 NH3 to the partial pressure of NH3 gas in contact with the liquid is a constant = 0.0173 (Henry’s Law Constant). This also says [NH3(aq)] =0.0173PNH3 or -1 PNH3 = 0.0173 [NH3(aq)] = 57.8 atm/M x [NH3(aq)] The latter form is like Table 5.1 except it has NH3 concentration in M instead of XNH3. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Producing Nitrogen Via Pressure Swing Adsorption
Reactions and Separations Producing Nitrogen via Pressure Swing Adsorption Svetlana Ivanova Pressure swing adsorption (PSA) can be a Robert Lewis Air Products cost-effective method of onsite nitrogen generation for a wide range of purity and flow requirements. itrogen gas is a staple of the chemical industry. effective, and convenient for chemical processors. Multiple Because it is an inert gas, nitrogen is suitable for a nitrogen technologies and supply modes now exist to meet a Nwide range of applications covering various aspects range of specifications, including purity, usage pattern, por- of chemical manufacturing, processing, handling, and tability, footprint, and power consumption. Choosing among shipping. Due to its low reactivity, nitrogen is an excellent supply options can be a challenge. Onsite nitrogen genera- blanketing and purging gas that can be used to protect valu- tors, such as pressure swing adsorption (PSA) or membrane able products from harmful contaminants. It also enables the systems, can be more cost-effective than traditional cryo- safe storage and use of flammable compounds, and can help genic distillation or stored liquid nitrogen, particularly if an prevent combustible dust explosions. Nitrogen gas can be extremely high purity (e.g., 99.9999%) is not required. used to remove contaminants from process streams through methods such as stripping and sparging. Generating nitrogen gas Because of the widespread and growing use of nitrogen Industrial nitrogen gas can be produced by either in the chemical process industries (CPI), industrial gas com- cryogenic fractional distillation of liquefied air, or separa- panies have been continually improving methods of nitrogen tion of gaseous air using adsorption or permeation. -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Dissolved Oxygen in the Blood
Chapter 1 – Dissolved Oxygen in the Blood Say we have a volume of blood, which we’ll represent as a beaker of fluid. Now let’s include oxygen in the gas above the blood (represented by the green circles). The oxygen exerts a certain amount of partial pressure, which is a measure of the concentration of oxygen in the gas (represented by the pink arrows). This pressure causes some of the oxygen to become dissolved in the blood. If we raise the concentration of oxygen in the gas, it will have a higher partial pressure, and consequently more oxygen will become dissolved in the blood. Keep in mind that what we are describing is a dynamic process, with oxygen coming in and out of the blood all the time, in order to maintain a certain concentration of dissolved oxygen. This is known as a) low partial pressure of oxygen b) high partial pressure of oxygen dynamic equilibrium. As you might expect, lowering the oxygen concentration in the gas would lower its partial pressure and a new equilibrium would be established with a lower dissolved oxygen concentration. In fact, the concentration of DISSOLVED oxygen in the blood (the CdO2) is directly proportional to the partial pressure of oxygen (the PO2) in the gas. This is known as Henry's Law. In this equation, the constant of proportionality is called the solubility coefficient of oxygen in blood (aO2). It is equal to 0.0031 mL / mmHg of oxygen / dL of blood. With these units, the dissolved oxygen concentration must be measured in mL / dL of blood, and the partial pressure of oxygen must be measured in mmHg. -

CEE 370 Environmental Engineering Principles Henry's
CEE 370 Lecture #7 9/18/2019 Updated: 18 September 2019 Print version CEE 370 Environmental Engineering Principles Lecture #7 Environmental Chemistry V: Thermodynamics, Henry’s Law, Acids-bases II Reading: Mihelcic & Zimmerman, Chapter 3 Davis & Masten, Chapter 2 Mihelcic, Chapt 3 David Reckhow CEE 370 L#7 1 Henry’s Law Henry's Law states that the amount of a gas that dissolves into a liquid is proportional to the partial pressure that gas exerts on the surface of the liquid. In equation form, that is: C AH = K p A where, CA = concentration of A, [mol/L] or [mg/L] KH = equilibrium constant (often called Henry's Law constant), [mol/L-atm] or [mg/L-atm] pA = partial pressure of A, [atm] David Reckhow CEE 370 L#7 2 Lecture #7 Dave Reckhow 1 CEE 370 Lecture #7 9/18/2019 Henry’s Law Constants Reaction Name Kh, mol/L-atm pKh = -log Kh -2 CO2(g) _ CO2(aq) Carbon 3.41 x 10 1.47 dioxide NH3(g) _ NH3(aq) Ammonia 57.6 -1.76 -1 H2S(g) _ H2S(aq) Hydrogen 1.02 x 10 0.99 sulfide -3 CH4(g) _ CH4(aq) Methane 1.50 x 10 2.82 -3 O2(g) _ O2(aq) Oxygen 1.26 x 10 2.90 David Reckhow CEE 370 L#7 3 Example: Solubility of O2 in Water Background Although the atmosphere we breathe is comprised of approximately 20.9 percent oxygen, oxygen is only slightly soluble in water. In addition, the solubility decreases as the temperature increases. -

Getting the Other Gas Laws
Getting the other gas laws If temperature is constant If volume is constant you you get Boyle’s Law get Gay-Lussac’s Law P1 P2 P1V1 = P2V2 = T1 T2 If pressure is constant you get Charles’s Law If all are variable, you get the Combined Gas Law. V V2 1 = P1V1 P2V2 T1 T2 = T1 T2 Boyle’s Law: Inverse relationship between Pressure and Volume. P1V1 = P2V2 P V Combined Gas Law: Gay-Lussac’s Law: P V P V Charles’ Law: 1 1 = 2 2 Direct relationship between T1 T2 Direct relationship between Temperature and Pressure. Temperature and Volume. P P V V 1 = 2 1 = 2 T1 T2 T1 T2 (T must be in Kelvin) T Dalton’s Law of Partial Pressures He found that in the absence of a chemical reaction, the pressure of a gas mixture is the sum of the individual pressures of each gas alone The pressure of each gas in a mixture is called the partial pressure of that gas Dalton’s law of partial pressures the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases The law is true regardless of the number of different gases that are present Dalton’s law may be expressed as PT = P1 + P2 + P3 +… Dalton’s Law of Partial Pressures What is the total pressure in the cylinder? Ptotal in gas mixture = P1 + P2 + ... Dalton’s Law: total P is sum of pressures. Gases Collected by Water Displacement Gases produced in the laboratory are often collected over water Dalton’s Law of Partial Pressures The gas produced by the reaction displaces the water, which is more dense, in the collection bottle You can apply Dalton’s law of partial -

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -

PRESSURE SWING ADSORPTION in the UNIT OPERATIONS LABORATORY Jason C
ChE laboratory PRESSURE SWING ADSORPTION IN THE UNIT OPERATIONS LABORATORY Jason C. Ganley Colorado School of Mines • Golden, CO 80401 ressure swing adsorption (PSA) is an industrial process adsorption occurs. The selection of a particular type of ad- typically used for the bulk separation of gas mixtures. sorbent may allow one component of a gas mixture to be An outgrowth of temperature swing adsorption, PSA preferentially adsorbed, the nature of surface and/or pore dif- Pis one of only a few gas-surface adsorption processes that fusion effects may vary for each gas in the mixture, and so on. allows for the separation of mixtures of gaseous species or PSA is a cyclic operation, and generally involves separa- vapors that exist in relatively high (non-trace) concentra- tion of a gas mixture by taking advantage of differences in tions in respect to one another. PSA has also been coupled adsorption thermodynamics or in diffusion rates that exist for to distillation processes for the separation of alcohol-water its various components. Cycling between higher and lower [1] vapor azeotropes. pressures allows components of the gas mixture to be removed It is interesting to note that, despite serving an important from (and later released to) the gas phase over designated pe- role for many decades in the chemical process industry, PSA riods of time. Students in the Unit Operations Laboratory may is not a central topic discussed in most chemical engineering consider the PSA cycle primarily as a mass transfer (rather educational resources, which instead focus on mass transfer than heat transfer) experiment. -

Raoult's Law – Partition Law
BAE 820 Physical Principles of Environmental Systems Henry’s Law - Raoult's Law – Partition law Dr. Zifei Liu Biological and Agricultural Engineering Henry's law • At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. Pi = KHCi • Where Pi is the partial pressure of the gaseous solute above the solution, C is the i William Henry concentration of the dissolved gas and KH (1774-1836) is Henry’s constant with the dimensions of pressure divided by concentration. KH is different for each solute-solvent pair. Biological and Agricultural Engineering 2 Henry's law For a gas mixture, Henry's law helps to predict the amount of each gas which will go into solution. When a gas is in contact with the surface of a liquid, the amount of the gas which will go into solution is proportional to the partial pressure of that gas. An equivalent way of stating the law is that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. the solubility of gases generally decreases with increasing temperature. A simple rationale for Henry's law is that if the partial pressure of a gas is twice as high, then on the average twice as many molecules will hit the liquid surface in a given time interval, Biological and Agricultural Engineering 3 Air-water equilibrium Dissolution Pg or Cg Air (atm, Pa, mol/L, ppm, …) At equilibrium, Pg KH = Caq Water Caq (mol/L, mole ratio, ppm, …) Volatilization Biological and Agricultural Engineering 4 Various units of the Henry’s constant (gases in water at 25ºC) Form of K =P/C K =C /P K =P/x K =C /C equation H, pc aq H, cp aq H, px H, cc aq gas Units L∙atm/mol mol/(L∙atm) atm dimensionless -3 4 -2 O2 769 1.3×10 4.26×10 3.18×10 -4 4 -2 N2 1639 6.1×10 9.08×10 1.49×10 -2 3 CO2 29 3.4×10 1.63×10 0.832 Since all KH may be referred to as Henry's law constants, we must be quite careful to check the units, and note which version of the equation is being used. -
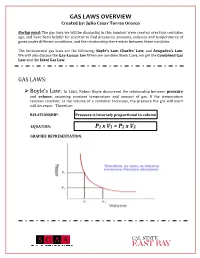
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant.