Introduction Boyle's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Pressure Diffusion Waves in Porous Media
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title Pressure diffusion waves in porous media Permalink https://escholarship.org/uc/item/5bh9f6c4 Authors Silin, Dmitry Korneev, Valeri Goloshubin, Gennady Publication Date 2003-04-08 eScholarship.org Powered by the California Digital Library University of California Pressure diffusion waves in porous media Dmitry Silin* and Valeri Korneev, Lawrence Berkeley National Laboratory, Gennady Goloshubin, University of Houston Summary elastic porous medium. Such a model results in a parabolic pressure diffusion equation. Its validity has been Pressure diffusion wave in porous rocks are under confirmed and “canonized”, for instance, in transient consideration. The pressure diffusion mechanism can pressure well test analysis, where it is used as the main tool provide an explanation of the high attenuation of low- since 1930th, see e.g. Earlougher (1977) and Barenblatt et. frequency signals in fluid-saturated rocks. Both single and al., (1990). The basic assumptions of this model make it dual porosity models are considered. In either case, the applicable specifically in the low-frequency range of attenuation coefficient is a function of the frequency. pressure fluctuations. Introduction Theories describing wave propagation in fluid-bearing porous media are usually derived from Biot’s theory of poroelasticity (Biot 1956ab, 1962). However, the observed high attenuation of low-frequency waves (Goloshubin and Korneev, 2000) is not well predicted by this theory. One of possible reasons for difficulties in detecting Biot waves in real rocks is in the limitations imposed by the assumptions underlying Biot’s equations. Biot (1956ab, 1962) derived his main equations characterizing the mechanical motion of elastic porous fluid-saturated rock from the Hamiltonian Principle of Least Action. -

What Is High Blood Pressure?
ANSWERS Lifestyle + Risk Reduction by heart High Blood Pressure BLOOD PRESSURE SYSTOLIC mm Hg DIASTOLIC mm Hg What is CATEGORY (upper number) (lower number) High Blood NORMAL LESS THAN 120 and LESS THAN 80 ELEVATED 120-129 and LESS THAN 80 Pressure? HIGH BLOOD PRESSURE 130-139 or 80-89 (HYPERTENSION) Blood pressure is the force of blood STAGE 1 pushing against blood vessel walls. It’s measured in millimeters of HIGH BLOOD PRESSURE 140 OR HIGHER or 90 OR HIGHER mercury (mm Hg). (HYPERTENSION) STAGE 2 High blood pressure (HBP) means HYPERTENSIVE the pressure in your arteries is higher CRISIS HIGHER THAN 180 and/ HIGHER THAN 120 than it should be. Another name for (consult your doctor or immediately) high blood pressure is hypertension. Blood pressure is written as two numbers, such as 112/78 mm Hg. The top, or larger, number (called Am I at higher risk of developing HBP? systolic pressure) is the pressure when the heart There are risk factors that increase your chances of developing HBP. Some you can control, and some you can’t. beats. The bottom, or smaller, number (called diastolic pressure) is the pressure when the heart Those that can be controlled are: rests between beats. • Cigarette smoking and exposure to secondhand smoke • Diabetes Normal blood pressure is below 120/80 mm Hg. • Being obese or overweight If you’re an adult and your systolic pressure is 120 to • High cholesterol 129, and your diastolic pressure is less than 80, you have elevated blood pressure. High blood pressure • Unhealthy diet (high in sodium, low in potassium, and drinking too much alcohol) is a systolic pressure of 130 or higher,or a diastolic pressure of 80 or higher, that stays high over time. -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Pressure Vs. Volume and Boyle's
Pressure vs. Volume and Boyle’s Law SCIENTIFIC Boyle’s Law Introduction In 1642 Evangelista Torricelli, who had worked as an assistant to Galileo, conducted a famous experiment demonstrating that the weight of air would support a column of mercury about 30 inches high in an inverted tube. Torricelli’s experiment provided the first measurement of the invisible pressure of air. Robert Boyle, a “skeptical chemist” working in England, was inspired by Torricelli’s experiment to measure the pressure of air when it was compressed or expanded. The results of Boyle’s experiments were published in 1662 and became essentially the first gas law—a mathematical equation describing the relationship between the volume and pressure of air. What is Boyle’s law and how can it be demonstrated? Concepts • Gas properties • Pressure • Boyle’s law • Kinetic-molecular theory Background Open end Robert Boyle built a simple apparatus to measure the relationship between the pressure and volume of air. The apparatus ∆h ∆h = 29.9 in. Hg consisted of a J-shaped glass tube that was Sealed end 1 sealed at one end and open to the atmosphere V2 = /2V1 Trapped air (V1) at the other end. A sample of air was trapped in the sealed end by pouring mercury into Mercury the tube (see Figure 1). In the beginning of (Hg) the experiment, the height of the mercury Figure 1. Figure 2. column was equal in the two sides of the tube. The pressure of the air trapped in the sealed end was equal to that of the surrounding air and equivalent to 29.9 inches (760 mm) of mercury. -

High Blood Pressure: Weight Control
Sacramento Heart & Vascular Medical Associates February 18, 2012 500 University Ave. Sacramento, CA 95825 Page 1 916-830-2000 Fax: 916-830-2001 Patient Information For: Only A Test High Blood Pressure: Weight Control What is high blood pressure? Blood pressure is the force of blood against artery walls as the heart pumps blood through the body. High blood pressure (hypertension) is blood pressure that keeps being higher than normal. How is high blood pressure affected by weight? One of the most important causes of high blood pressure is overweight. An unhealthy weight puts stress on the heart and lungs, forcing them to work harder. Losing weight reduces the stress on your heart. It can also lower your blood pressure. What can I do to control my weight? Change your eating habits so that you lose 1 to 2 pounds a week until you reach a healthy weight. Even a modest weight loss of 5 to 10 pounds can help. Your diet needs to be low in fat, cholesterol, and salt. Be careful about serving sizes. Don't drink a lot of juice or soda. Also limit the amount of alcohol you drink. A regular, moderate exercise program can help you lose weight or keep a normal weight because it increases your metabolism and burns up calories. It reduces stress and promotes good health. Exercise also lowers your cholesterol and blood sugar levels. Ask your healthcare provider to recommend a diet and exercise program that is right for you. How long will the effects last? If you are overweight and have high blood pressure, you will need to control your blood pressure all of your life. -

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -

Quick Reference Guide on the Units of Measure in Hyperbaric Medicine
Quick Reference Guide on the Units of Measure in Hyperbaric Medicine This quick reference guide is excerpted from Dr. Eric Kindwall’s chapter “The Physics of Diving and Hyperbaric Pressures” in Hyperbaric Medicine Practice, 3rd edition. www.BestPub.com All Rights Reserved 25 THE PHYSICS OF DIVING AND HYPERBARIC PRESSURES Eric P. Kindwall Copyright Best Publishing Company. All Rights Reserved. www.BestPub.com INTRODUCTION INTRODUCTIONThe physics of diving and hyperbaric pressure are very straight forTheward physicsand aofr edivingdefi nanded hyperbaricby well-k npressureown a nared veryacce pstraightforwardted laws. Ga sandu narede r definedpres subyr ewell-knowncan stor eande nacceptedormous laws.ene rgGasy, underthe apressuremount scanof storewhi cenormoush are o fenergy,ten the samountsurprisin ofg. whichAlso, s arema loftenl chan surprising.ges in the pAlso,erce smallntage schangesof the inva rtheiou spercentagesgases use dofa there variousgrea gasestly m usedagni fareied greatlyby ch amagnifiednges in am byb ichangesent pre sinsu ambientre. The pressure.resultan t pTheh yresultantsiologic physiologiceffects d effectsiffer w idifferdely d ewidelypend idependingng on the onp rtheess upressure.re. Thus ,Thus,divi ndivingg or oorpe operatingrating a a hy- perbarichype rfacilitybaric f requiresacility re gainingquires gcompleteaining c oknowledgemplete kn ofow theled lawsge o finvolvedthe law stoin ensurevolved safety.to ensure safety. UNITS OF MEASURE UNITS OF MEASURE This is often a confusing area to anyone new to hyperbaric medicine as both Ameri- This is often a confusing area to anyone new to hyperbaric medicine as can and International Standard of Units (SI) are used. In addition to meters, centimeters, both American and International Standard of Units (SI) are used. In addition kilos,to pounds,meters, andcen feet,time someters, kpressuresilos, po uarend givens, an dinf atmosphereseet, some p rabsoluteessures andare millimetersgiven in of mercury.atmosph Tableeres 1a bgivessolu thete a exactnd m conversionillimeters factorsof me rbetweencury. -

Pressure and Fluid Statics
cen72367_ch03.qxd 10/29/04 2:21 PM Page 65 CHAPTER PRESSURE AND 3 FLUID STATICS his chapter deals with forces applied by fluids at rest or in rigid-body motion. The fluid property responsible for those forces is pressure, OBJECTIVES Twhich is a normal force exerted by a fluid per unit area. We start this When you finish reading this chapter, you chapter with a detailed discussion of pressure, including absolute and gage should be able to pressures, the pressure at a point, the variation of pressure with depth in a I Determine the variation of gravitational field, the manometer, the barometer, and pressure measure- pressure in a fluid at rest ment devices. This is followed by a discussion of the hydrostatic forces I Calculate the forces exerted by a applied on submerged bodies with plane or curved surfaces. We then con- fluid at rest on plane or curved submerged surfaces sider the buoyant force applied by fluids on submerged or floating bodies, and discuss the stability of such bodies. Finally, we apply Newton’s second I Analyze the rigid-body motion of fluids in containers during linear law of motion to a body of fluid in motion that acts as a rigid body and ana- acceleration or rotation lyze the variation of pressure in fluids that undergo linear acceleration and in rotating containers. This chapter makes extensive use of force balances for bodies in static equilibrium, and it will be helpful if the relevant topics from statics are first reviewed. 65 cen72367_ch03.qxd 10/29/04 2:21 PM Page 66 66 FLUID MECHANICS 3–1 I PRESSURE Pressure is defined as a normal force exerted by a fluid per unit area. -

High Blood Pressure
HIGH BLOOD PRESSURE BLOOD PRESSURE is a measure of how hard your blood pushes against your arteries as it moves through your body. Blood pressure rises and falls naturally during the day. But if it stays too high, over time it can lead to health problems, such as a heart attack, stroke or heart failure. High blood pressure is also called hypertension. Often, there may be no signs or symptoms that tell you when your blood pressure is too high. The good news: High blood pressure can be treated or even prevented. What the Numbers Mean Lifestyle changes are the main treatment for those with elevated (120-129 mmHg/<80 Blood pressure is given as two numbers. mmHg) or stage 1 high blood pressure (130- Systolic blood pressure is the top number, 139 mmHg/80-89 mmHg). They are also and diastolic blood pressure is the bottom important in treating individuals with stage 2 number. Under new guidelines, high blood high blood pressure (>140 mmHG/>90 mmHG) pressure is now defined as 130 mmHg/80 and for good health overall. mmHg or greater. Normal blood pressure is less than 120 mmHg/80 mmHg. Lifestyle Changes to Lower Research shows that lower blood pressure Your Blood Pressure goals improve heart health. Lose weight, if needed, and maintain a healthy body weight. Focus on healthy eating: Follow the Dietary Approaches to Stop Hypertension (DASH) diet, high in fruits, vegetables, and low-fat dairy. Cut the amount of salt (sodium) you eat. For people with high blood pressure or at risk for it, less than HOW MUCH 1,500 mg per day is often the goal. -

Marine Tourism Impacts and Their Management on the Great Barrier Reef
Marine tourism impacts and their management on the Great Barrier Reef VJ Harriott CRC Reef Research Centre & James Cook University www.reef.crc.org.au Established and supported under the Australian Government’s Cooperative Research Centres Program CRC REEF RESEARCH CENTRE TECHNICAL REPORT NO 46 CRC Reef Research Centre Ltd is a joint venture between: Association of Marine Park Tourism Operators, Australian Institute of Marine Science, Great Barrier Reef Marine Park Authority, Great Barrier Reef Research Foundation, James Cook University, Queensland Department of Primary industries, Queensland Seafood Industry Association and Sunfish Queensland Inc. CRC REEF RESEARCH CENTRE TECHNICAL REPORT NO. 46 MARINE TOURISM IMPACTS AND THEIR MANAGEMENT ON THE GREAT BARRIER REEF Vicki J. Harriott CRC Reef Research Centre and School of Tropical Environmental Science and Geography, James Cook University. A report funded by the CRC Reef Research Centre. The CRC Reef Research Centre was established under the Australian Government’s Cooperative Research Centres Program. It is a knowledge-based partnership of coral reef managers, researchers and industry. Its mission is to plan, fund and manage world-leading science for the sustainable use of the Great Barrier Reef World Heritage Area. Partner organisations are: · Association of Marine Park Tourism Operators · Australian Institute of Marine Science · Great Barrier Reef Marine Park Authority · Great Barrier Reef Research Foundation · James Cook University · Queensland Department of Primary Industries · Queensland Seafood Industry Association · SUNFISH Queensland Inc. CRC Reef Research Centre PO Box 772 Townsville QLD 4810 Australia Phone: 07 4729 8400 Fax: 07 4729 8499 Email: [email protected] Web: www.reef.crc.org.au CRC Reef Research Centre Technical Report No. -
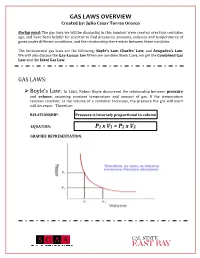
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant.