PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction Day To
Cycling over the Veluwe - 6 dagen DUTCH BIKETOURS - EMAIL: [email protected] - TELEPHONE +31 (0)24 3244712 - WWW.DUTCH-BIKETOURS.COM Cycling over the Veluwe 6 days, € 410 Introduction This tour has been designed for cyclists who want to truly live life to the full. During the day, you cycle through beautiful natural scenery and in the evenings you stay at Westcord Hotel de Veluwe in the village of Garderen. For no fewer than six days - what a treat! Westcord Hotel de Veluwe is known for its comfort, culinary qualities and hospitality. Your cycling holiday has never been so comfortable! The routes surprise you with a wealth of natural beauty and interesting sights. You can visit the Hoge Veluwe National Park with the Kröller-Muller museum, the Royal Palace Het Loo or the Hanseatic city of Harderwijk. During your stay, you can choose from 4 different bicycle routes. Each day you will have 3 distances to choose from. Day to Day Day 1 Arrival at Garderen Your hotel is situated in a unique part of the Netherlands: the centre of The Veluwe. Today, you have plenty of time to take in the beautiful surroundings. The welcoming village of Garderen is certainly worth a visit. Day 2 Route Northern Veluwe 56 km Vast forests, purple-colored heather fields, picturesque towns and charming cities: with the Noord Veluwe route you can see it all. You cycle over the Ermelose Heide, a heathland of no less than 343 hectares. You may encounter a shepherd with his sheepfold on the way. The largest sheep herd in Europe is located on the Ermelose heath. -

Chartmaking in England and Its Context, 1500–1660
58 • Chartmaking in England and Its Context, 1500 –1660 Sarah Tyacke Introduction was necessary to challenge the Dutch carrying trade. In this transitional period, charts were an additional tool for The introduction of chartmaking was part of the profes- the navigator, who continued to use his own experience, sionalization of English navigation in this period, but the written notes, rutters, and human pilots when he could making of charts did not emerge inevitably. Mariners dis- acquire them, sometimes by force. Where the navigators trusted them, and their reluctance to use charts at all, of could not obtain up-to-date or even basic chart informa- any sort, continued until at least the 1580s. Before the tion from foreign sources, they had to make charts them- 1530s, chartmaking in any sense does not seem to have selves. Consequently, by the 1590s, a number of ship- been practiced by the English, or indeed the Scots, Irish, masters and other practitioners had begun to make and or Welsh.1 At that time, however, coastal views and plans sell hand-drawn charts in London. in connection with the defense of the country began to be In this chapter the focus is on charts as artifacts and made and, at the same time, measured land surveys were not on navigational methods and instruments.4 We are introduced into England by the Italians and others.2 This lack of domestic production does not mean that charts I acknowledge the assistance of Catherine Delano-Smith, Francis Her- and other navigational aids were unknown, but that they bert, Tony Campbell, Andrew Cook, and Peter Barber, who have kindly commented on the text and provided references and corrections. -
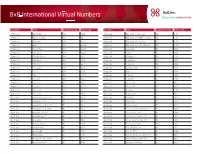
International Rate Centers for Virtual Numbers
8x8 International Virtual Numbers Country City Country Code City Code Country City Country Code City Code Argentina Bahia Blanca 54 291 Australia Brisbane North East 61 736 Argentina Buenos Aires 54 11 Australia Brisbane North/North West 61 735 Argentina Cordoba 54 351 Australia Brisbane South East 61 730 Argentina Glew 54 2224 Australia Brisbane West/South West 61 737 Argentina Jose C Paz 54 2320 Australia Canberra 61 261 Argentina La Plata 54 221 Australia Clayton 61 385 Argentina Mar Del Plata 54 223 Australia Cleveland 61 730 Argentina Mendoza 54 261 Australia Craigieburn 61 383 Argentina Moreno 54 237 Australia Croydon 61 382 Argentina Neuquen 54 299 Australia Dandenong 61 387 Argentina Parana 54 343 Australia Dural 61 284 Argentina Pilar 54 2322 Australia Eltham 61 384 Argentina Rosario 54 341 Australia Engadine 61 285 Argentina San Juan 54 264 Australia Fremantle 61 862 Argentina San Luis 54 2652 Australia Herne Hill 61 861 Argentina Santa Fe 54 342 Australia Ipswich 61 730 Argentina Tucuman 54 381 Australia Kalamunda 61 861 Australia Adelaide City Center 61 871 Australia Kalkallo 61 381 Australia Adelaide East 61 871 Australia Liverpool 61 281 Australia Adelaide North East 61 871 Australia Mclaren Vale 61 872 Australia Adelaide North West 61 871 Australia Melbourne City And South 61 386 Australia Adelaide South 61 871 Australia Melbourne East 61 388 Australia Adelaide West 61 871 Australia Melbourne North East 61 384 Australia Armadale 61 861 Australia Melbourne South East 61 385 Australia Avalon Beach 61 284 Australia Melbourne -

Aan De Leden Van De Gemeenteraden Van Elburg, Ermelo, Harderwijk, Hattem, Nunspeet, Oldebroek En Putten
Aan de leden van de gemeenteraden van Elburg, Ermelo, Harderwijk, Hattem, Nunspeet, Oldebroek en Putten Datum: 11 april 2017 Onderwerp : Informatie over voortgang transitie van RNV naar nieuwe samenwerking Noord-Veluwe. Geacht raadslid, Met deze brief informeren wij u tussentijds over de voortgang van de transitie van RNV naar de nieuwe samenwerking Noord-Veluwe. Hierna komen achtereenvolgens de volgende onderwerpen aan bod: 1. Hoofdpunten uit collegeconferenties 21 februari en 28 maart 2017. 2. Conceptversie ambtelijk voorstel verdeling ambtelijke capaciteit Samenwerking Noord-Veluwe. 3. Uitvoeringsplan voor opbouw nieuwe samenwerking. 4. Betrokkenheid Raden in komende periode. 1. Hoofdpunten uit collegeconferentie 21 februari en 28 maart 2017 Nadat de Kwartiermaker begin januari 2017 zijn eindrapportage “De kunst van het verbinden” aan de gemeenten heeft aangeboden, is gestart met de voorbereiding van de afbouw van RNV en de vormgeving van de nieuwe samenwerking. Op 21 februari 2017 vond een eerste collegeconferentie plaats, waaraan de colleges Elburg, Ermelo, Harderwijk, Hattem, Nunspeet, Oldebroek en Putten deelnamen. Uitgangspunten voor de nieuwe samenwerking Doelstelling van de bijeenkomst op 21 februari was het formuleren van uitgangspunten en voorwaarden voor een succesvolle vernieuwde samenwerking. Over de volgende onderwerpen zijn nadere afspraken gemaakt: • De invulling van de politiek-inhoudelijke verantwoordelijkheid van vakwethouders in de nieuwe samenwerking; • De verwachtingen van de rolinvulling van de Regiegroep (voorheen de Stuurgroep) (de Regiegroep staat onder voorzitterschap van de burgemeester van Harderwijk en bestaat daarnaast uit de burgemeesters van Elburg, Ermelo, Hattem, Nunspeet, Oldebroek en Putten en de wethouder van Harderwijk); • Waarborgen van waardevolle kenmerken in de huidige samenwerking in de nieuwe samenwerking; • De betekenis van alternatieve regio-indelingen voor de deelname van bepaalde gemeenten aan bepaalde overleggen. -

The Path to the FAIR HANSA FAIR for More Than 600 Years, a Unique Network HANSA of Merchants Existed in Northern Europe
The path to the FAIR HANSA FAIR For more than 600 years, a unique network HANSA of merchants existed in Northern Europe. The cooperation of this consortium of merchants for the promotion of their foreign trade gave rise to an association of cities, to which around 200 coastal and inland cities belonged in the course of time. The Hanseatic League in the Middle Ages These cities were located in an area that today encom- passes seven European countries: from the Dutch Zui- derzee in the west to Baltic Estonia in the east, and from Sweden‘s Visby / Gotland in the north to the Cologne- Erfurt-Wroclaw-Krakow perimeter in the south. From this base, the Hanseatic traders developed a strong economic in uence, which during the 16th century extended from Portugal to Russia and from Scandinavia to Italy, an area that now includes 20 European states. Honest merchants – Fair Trade? Merchants, who often shared family ties to each other, were not always fair to producers and craftsmen. There is ample evidence of routine fraud and young traders in far- ung posts who led dissolute lives. It has also been proven that slave labor was used. ̇ ̆ Trading was conducted with goods that were typically regional, and sometimes with luxury goods: for example, wax and furs from Novgorod, cloth, silver, metal goods, salt, herrings and Chronology: grain from Hanseatic cities such as Lübeck, Münster or Dortmund 12th–14th Century - “Kaufmannshanse”. Establishment of Hanseatic trading posts (Hanseatic kontors) with common privi- leges for Low German merchants 14th–17th Century - “Städtehanse”. Cooperation between the Hanseatic cit- ies to defend their trade privileges and Merchants from di erent cities in di erent enforce common interests, especially at countries formed convoys and partnerships. -

Zuiderzeestraatweg: Het Verhaal
Deel 1 De Zuiderzeestraatweg: Het Verhaal Een gewone weg met een bijzondere betekenis voor de noordelijke Veluwezoom Deel 1 De Zuiderzeestraatweg: Het Verhaal Colofon Dit project werd uitgevoerd door de Regio Noord Veluwe in samenwerking met het Gelders Genootschap. Het project kwam tot stand met medewerking en financiering van de Provincie Gelderland, het Veluws Bureau voor Toerisme en de gemeenten Nijkerk, Putten, Ermelo, Harderwijk, Nunspeet, Elburg, Oldebroek en Hattem. Auteur: Jan Wabeke Maart 2011 Gelders Genootschap / Regio Noord Veluwe Gelders Genootschap Vereniging tot bevordering en instandhouding van de schoonheid van stad en land Zypendaalseweg 46 Postbus 68 6800 AB Arnhem T: 026-4421742 www.geldersgenootschap.nl 2 Zuiderzeestraatweg Een gewone weg met een bijzondere betekenis voor de noordelijke Veluwezoom Deel 1 Het verhaal | Maart 2011 | Gelders Genootschap 3 Inhoudsopgave De rapportage van Het project Zuiderzeestraatweg Deel 1 bestaat uit 3 delen: De Zuiderzeestraatweg: Het Verhaal Deel 1: De Zuiderzeestraatweg: Het Verhaal: 1. Voorgeschiedenis 10 Hoe is de weg tot stand gekomen? Over welke weg 2. Aanleg en ontwikkeling 17 hebben we het? Wat waren oudere wegen in het 3. Landschap en bebouwing 25 gebied? Hoe heeft de weg zich ontwikkeld? En hoe is de omgeving veranderd? Door welke landschappen voert Bronnen 29 de weg? En wat voor bijzondere bebouwing komen we tegen onderweg? Dit zijn vragen die in dit deel aan de aan de orde komen, naast verhalen over bijzondere gebeurtenissen die te maken hebben met de straatweg. Deel 2: De Zuiderzeestraatweg: Het Streefbeeld: Naar welke belevingskwaliteit streven we voor de straatweg? Welke ontwerpprincipes worden gehanteerd om de weg een nieuwe grandeur te geven? Hoe kunnen we het streefbeeld realiseren? Deel twee gaat in op deze vragen. -

1926-72-215.Pdf
135 136 2° in crastino beati Pontiani (15 Jan.) 1317 als de 7°. Reinera, ged. te Nunspeet 3 Dec. 1637 overl. Bisschop van Utrecht uitspraak doet in de zaak tusschen vóór 1671, huwde te Gorssel 10 Juni 1666 (o. te Steven van Wede voor zijn zusters Agnese en Mechtelt Deventer 19, te Harderwijk 20 Mei, van Deven• eenerzijds en heer Borre, zijn broeder Diderïlc Borre ter naar Gorssel geatt. 10 Juni): Berent Dapper, en Jan van Amerongen anderzijds, over de uitkeering zn. van Bitier Dapper en Christina Elsinck, ged. van 200 ffi, of 20 ffi jaarlijks, zwarte tornoisen aan Agnese te Deventer 27 Sept. 1640, gemeensman te Deven• en Mechtelt uit de goederen, die hun zuster Jutte geërfd ter voor de Assonstraat 1681—1702, provisor, had van haar man Hughe van Dorsghen en hun kind. begr. te Deventer 27 Juni 1707. Hij hertr. met Na verklaring van de getuigen bij het huwelijk van Anna Jacobsen. Jutte met Jan van Woudenberg, zoon van heer Borre vd., 8°. Ds. Eilardus, ged. te Nunspeet 21 Juni 1640, wordt aan Agnese en Mechtelt die uitkeering toegekend. ingeschr. als student te Groningen 13 Sept. 1660, (Regr nr 206, p. 75; Mr. Muller, nr 398). predikant te Putten 1665, overl to Putton 8 Nov, Hieruit leeren wij 3 zusters van Steven van Weede 1704, ongehuwd. kennen, zonder dat wij nochtans iets vinden mochten Ilbis. Ds. Samuel Verslege, geb. te Buren, ingeschr. over hun ouders. als student te Groningen 28 Mei 1622, te Leiden 2L Sept. 1623, predikant te Didam 1627, te Warns- veld 1629, overl. aan de pest 27 Aug. -

Overzicht Alle Beschreven Panden Deventer
Beschrijving van panden Naam Straat Huisnr Info Algemene informatie Deze en andere beschrijvingen (met wandelkaart) van stadswandelingen in de Hanzesteden vindt u via www.wandelenindeventer.nl. Harderwijk De lengte van de wandeling bedraagt ongeveer 4 km. Harderwijk bezit als oude Hanzestad een rijke erfenis.In 1223, het jaar waarin Harderwijk voor het eerst werd genoemd, was de plaats al van een agrarische nederzetting uitgegroeid tot handelsplaats. Rond 1500 was in Harderwijk de belangrijkste vismarkt van de Zuiderzee te vinden. Nog steeds herinneren de Vischpoort en andere monumenten aan deze periode in de geschiedenis. Linnaeus torentje Academiestraat Het Linnaeustorentje zat in de 16de eeuw vast aan het winterhuis van de prior van 's-Heerenloo, een instelling van de Johanniter Orde in Ermelo. De woning is door brand verwoest, maar het torentje bleef een markant punt waarin studenten die zich misdroegen moesten 'zitten' en op z'n minst claustrofobisch werden. De toegangsdeur is zo laag, dat je bij het betreden vanzelf het hoofd buigt - wat zal dat de universitaire rechtbank goed gedaan hebben. In het torentje staat het borstbeeld van de grote Zweedse botanicus en hangt een bronzen plaquette ter herinnering aan Boerhaaves bliksembezoek. Academiegebouw Academiestraat 14 Aanvankelijk heeft er op deze locatie een kostschool voor leerlingen gezeten. Het gebouw was later van de Gelderse Academie. Boven de deur staat het Harderwijk jaartal 1754. Veel later was er de sociale dienst gevestigd. De blauwe hardstenen stoep kwam voor in een lokaal gezegde… als op de 'Blauwe stoep' was gesignaleerd dan wist een ieder hoe je er financieel voor stond. woensdag 27 juli 2011 Page 1 of 9 Naam Straat Huisnr Info Vanghentoren Blokhuis 11 Op 29 april 1310 kocht de graaf van Gelre van de Harderwijker magistraat een stuk grond buiten de stadsmuur om daar ‘zijn huis’ te bouwen. -

Statistical Yearbook of the Netherlands 2004
Statistical Yearbook of the Netherlands 2004 Statistics Netherlands Preface Statistics Netherlands has a long tradition in the publication of annual figures and yearbooks. The Statistical Yearbook has been the most popular publication by Statistics Netherlands for decades. This latest edition again provides facts and figures on virtually all aspects of Dutch society. It is an invaluable resource for a quick exploration of the economy, population issues, education, health care, crime, culture, the environment, housing, and many other topics. This year’s volume is structured in exactly the same way as last year. It contains the data available at the end of November 2003. For current updates please check the Statline Database at Statistics Netherlands, which is in the process of being translated into English. It can be accessed free of charge at www.cbs.nl. G. van der Veen Director General of Statistics Voorburg / Heerlen, April 2004 Preface Statistical Yearbook 2004 3 Published by Explanation of symbols Statistics Netherlands Prinses Beatrixlaan 428 . = figure not available 2273 XZ Voorburg * = provisional figure The Netherlands x = publication prohibited (confidential figure) Lay out – = nil Statistics Netherlands 0 (0.0) = less than half of unit concerned Facility services department blank = not applicable < = fewer / less / smaller than > = more / greater than Cover design ≤ = fewer / less / smaller than or equal to WAT ontwerpers (Utrecht) ≥ = more / greater than or equal to 2003-2004 = 2003 to 2004 inclusive Print 2003/2004 = average of 2003 up to and Opmeer | De Bink | TDS v.o.f., The Hague including 2004 2003/’04 = crop year, financial year, school Translation year etc. beginning in 2003 and Statistics Netherlands ending in 2004 Rita Gircour Due to rounding, some totals may not correspond with Information the sum of the separate figures E-mail [email protected] How to order Obtainable from The Sdu publishers P.O. -

Introduction Day To
Stagecoach Tour - 7 dagen DUTCH BIKETOURS - EMAIL: [email protected] - TELEPHONE +31 (0)24 3244712 - WWW.DUTCH-BIKETOURS.COM Stagecoach Tour 7 days, € 570 Introduction The lovely Stagecoach Route runs through attractive and diverse landscapes. The Posbank, for example, is part of National Park Veluwezoom. It’s an area of heath, pine trees and sand drifts. After a hefty climb to the top, enjoy breathtaking views. You’ll also visit a riverbank nature reserve as well as a string of pretty towns that used to be members of the Hansa League, a Northern European alliance of trading guilds in the 13th-17th Centuries. You will pass the former Hanseatic towns and cities such as Hattem, Zwolle, Deventer, Zutphen, Harderwijk and Elburg. Day to Day Day 1 Arrival in Ede Arrival in Ede Day 2 Ede - Strand Nulde 56 km From the hotel you leave Ede and go in the direction of Lunteren. Your first destination is the geographic center of the Netherlands at the Lunterse Goudsberg, where you cycle between woods, heathland, drifting sands and old farmland. If you opt for the standard (longer) route, you will find the so-called Celtic Fields, just past the drifting sand area Wek eromse Zand. The Celtic Fields are agricultural land from the Iron Age, with a reconstructed farm. Continue the route to the Veluwe region, with a mix of forest, heathland and sand with agricultural villages such as Kootwijk and Garderen in between. The shorter route of 47 km goes over Barneveld, once the junction of Hanze and Hessen weggen. This route meanders through a varied agricultural landscape interspersed with woods, heaths and peatlands and many hamlets such as Appel. -

Utrecht-Zwolle: Elk Kwartier Een Intercity
Impuls voor de Veluwelijn Meer directe verbindingen Groningen en Leeuwarden Utrecht-Zwolle: elk kwartier een intercity Notitie van het lid Slob lid van de fractie van de ChristenUnie in de Tweede Kamer der Staten – Generaal 7 oktober 2010 1 Inleiding In deze notitie doet de ChristenUnie-fractie een voorstel voor enkele relatief kleine ingrepen op de spoorlijn Utrecht-Zwolle (Veluwelijn) waardoor het aantal intercities zou kunnen verdubbelen. Doel van deze notitie is om te komen tot een kwartierdienst voor de intercity Utrecht-Zwolle zodat het noorden beter wordt aangesloten op het hoogfrequente spoorvervoer in de Randstad. Dit zou een enorme kwaliteitssprong kunnen betekenen op dit traject waar de treinen nu al op grote delen van de dag overvol zitten. Ondanks twee Kamermoties waarvan de eerste al dateert van zomer 2006 en diverse Kamervragen heeft de Kamer nog steeds geen onderzoek ontvangen wat er nodig is voor een dergelijke kwaliteitssprong. In deze notitie staat daarom een uitgewerkt voorstel wat kan dienen als vertrekpunt voor een door de minister uit te voeren planstudie. 2 Analyse knelpunt Momenteel rijden er 2 intercities en 2 sprinters per uur. Op de drukste uren van de dag rijden er 4 IC’s per uur. Deze intercities rijden echter vlak achter elkaar (zogenaamde voor- en volgtreinen van de reguliere intercity) gezien de beperkte spoorcapaciteit. Dit wordt veroorzaakt door het grote aantal stopstations tussen de twee IC stations Zwolle en Amersfoort. Dit past niet binnen de gewenste kwaliteit van het Programma Hoogfrequent Spoorvervoer waarbij de treinen goed gespreid zijn over het uur om zo meer reizigers te trekken. -

118739Pos.Pdf
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a postprint version which may differ from the publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/118739 Please be advised that this information was generated on 2021-09-27 and may be subject to change. “Long-term follow up of axillary recurrences after negative Sentinel Lymph Node Biopsy; effect on prognosis and survival” Running head: Axillary recurrences after negative SLNB J.P. Bulte, MD dep. of Surgery, Canisius Wilhelmina Hospital, Nijmegen and Radboud University Nijmegen Medical Centre, Nijmegen the Netherlands [email protected] B.J. van Wely, MD dep. of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands [email protected] S. Kasper, BSc dep of Surgery, Radboud University Nijmegen Medical Centre, Nijmegen the Netherlands [email protected] G. Kuijt, MD dep of Surgery, Maxima Medical Centre, Veldhoven the Netherlands [email protected] F.J.H. van den Wildenberg, MD dep. of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands [email protected] L.J.A. Strobbe, MD PhD dep. of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands [email protected] J.H.W. de Wilt, MD PhD dep. of Surgery, Radboud University Nijmegen Medical Centre, Nijmegen the Netherland [email protected] Corresponding author J.P. Bulte Department of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands Weg door jonkerbos 100, 6532SB Nijmegen, the Netherlands Telephone 0031 24 3658720 fax 0031 24 3658725 Keywords: Breast cancer, Sentinel lymph node biopsy, Axillary recurrence, prognosis 1 Abstract Background: As axillary recurrence (AR) after a negative sentinel lymph node biopsy (SLNB) is rare, the prognosis of these patients is unknown.