Combining High-Sensitivity Troponin with the AHA/ACC Cholesterol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
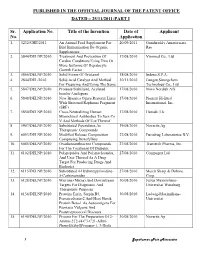
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

Recent Advances in Oligonucleotide Therapeutics in Oncology
International Journal of Molecular Sciences Review Recent Advances in Oligonucleotide Therapeutics in Oncology Haoyu Xiong 1, Rakesh N. Veedu 2,3 and Sarah D. Diermeier 1,* 1 Department of Biochemistry, University of Otago, Dunedin 9016, New Zealand; [email protected] 2 Centre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth 6150, Australia; [email protected] 3 Perron Institute for Neurological and Translational Science, Perth 6009, Australia * Correspondence: [email protected] Abstract: Cancer is one of the leading causes of death worldwide. Conventional therapies, including surgery, radiation, and chemotherapy have achieved increased survival rates for many types of cancer over the past decades. However, cancer recurrence and/or metastasis to distant organs remain major challenges, resulting in a large, unmet clinical need. Oligonucleotide therapeutics, which include antisense oligonucleotides, small interfering RNAs, and aptamers, show promising clinical outcomes for disease indications such as Duchenne muscular dystrophy, familial amyloid neuropathies, and macular degeneration. While no approved oligonucleotide drug currently exists for any type of cancer, results obtained in preclinical studies and clinical trials are encouraging. Here, we provide an overview of recent developments in the field of oligonucleotide therapeutics in oncology, review current clinical trials, and discuss associated challenges. Keywords: antisense oligonucleotides; siRNA; aptamers; DNAzymes; cancers Citation: Xiong, H.; Veedu, R.N.; 1. Introduction Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in According to the Global Cancer Statistics 2018, there were more than 18 million new Oncology. Int. J. Mol. Sci. 2021, 22, cancer cases and 9.6 million deaths caused by cancer in 2018 [1]. -

יומן הפטנטים והמדגמים Patents and Designs Journal
י /' התשס"ח 5/2008 רשומות ISRAEL STATE RECORDS ו' באב התשס"ח August 7, 2008 יומן הפטנטים והמדגמים PATENTS AND DESIGNS JOURNAL פטנטים עמוד PATENTS Page בקשות שהוגשו Applications filed 1507 בקשות שקובלו Applications accepted 1775 פטנטים שניתנו Patents granted 1992 פטנטים שחודשו Patents renewed 1993 פטנטים שתוקפם פקעו Patents not in force 1995 פטנטים שחודשו לעשרים שנה Patents renewed for 20 years 1996 פטנטים שפג תוקפם Patents expired 1997 הודעות Notices 1998 שינויים בפרטים רשומים Changes in particulars entered בפנקס in register 2001 תיקוני טעויות Corrigenda 2002 מפתחות לבקשות שקובלו Indices of applications accepted i מדגמים DESIGNS מדגמים שנרשמו Designs registered 2004 מדגמים שחודשו Designs renewed 2017 מדגמים שבוטלו Designs void 2018 ו' באב התשס"ח – August 7, 2008 1507 ידיעות כלליות מכתבים, מסמכים, וכו' בענייני פטנטים ומדגמים יש לשלוח אל: רשם הפטנטים והמדגמים, רח' הסדנא 4, ירושלים לשכת הפטנטים נמצאת ברח' הסדנא 4, תלפיות, ירושלים והיא פתוחה לציבור בימי חול שאינם ערבי שבת או מועד בין השעות 8:30 ו - 12:30. לשכת הפטנטים מספקת תצלומים של פירוטים ושרטוטים במחיר של 2.50 שקלים בעד כל עמוד או חלק ממנו. אגרות ללשכת הפטנטים מתקבלות אך ורק על ידי תשלום לחשבון הלשכה בבנק הדואר מס' 0-24145-2. יש להציג קבלת בנק הדואר ללשכה יחד עם הבקשה לפעולה שעבורה האגרה שולמה. GENERAL INFORMATION Letters, documents, etc. concerning Patents and Designs should be addressed to: The Commissioner of Patents and Designs, 4 Hasadnah St., Jerusalem The Patent Office is located at 4 Hasadnah St., Talpiot, Jerusalem and is open to the public on weekdays, except on Fridays or on the eves of holydays, from 08:30 to 12:30 hrs. -

Medical Research Council
Elan Pharmaceuticals University of Kansas Parke-Davis Basilea Pharmaceutica Office of Rare Diseases (ORD) Swedish Orphan Biovitrum Astellas Pharma US, Inc. Food and Drug Administration (FDA) Biogen Idec AVEO Pharmaceuticals, Inc. Bioclin Research Laboratories Rare Diseases Clinical Research Network Birmingham Clinical Trials Unit Astellas Pharma Inc Baylor College of Medicine Astellas Pharma Europe BV Abbott Biotherapeutics Corp. OSI Pharmaceuticals Anglo-European College of Chiropractic The Beverage Institute. National Development and Research Institutes, Inc. Boston University University of Rochester Medstar Research Institute Omnicare Clinical Research ABX-CRO Bio-Kinetic Europe Ltd MRC Human Nutrition Research, Cambridge, UK. Shionogi Astellas Pharma Global Development, Inc. Ethicon, Inc. Symphogen A/S Averion-Hesperion Quintiles Laboratories Temple University Emory University National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) European Chiropractors Union Children's Hospital Boston Colchester Hospital University NHS Foundation Trust Covance GE Healthcare EMSI National Institute for Chiropractic Research Muscular Dystrophy Association Phoenix Children's Hospital OMRIX Biopharmaceuticals University of California, Los Angeles Cancer and Leukemia Group B University Health Network, Toronto University of Iowa Sarcoma Alliance for Research through Collaboration Westat Medivation, Inc. International Breast Cancer Study Group National Institute of Neurological Disorders and Stroke (NINDS) ClinPhone, Inc. Southwest Oncology Group Multi-Imagem and CDPI, Rio de Janeiro, Brasil Dutch Cancer Society Gary Cutter, PhD Murdoch Childrens Research Institute Chiltern International Inc. Janssen Research & Development, LLC Birmingham Children's Hospital NHS Foundation Trust Southwestern Oncology Group (SWOG) CIHR Canadian HIV Trials Network Royal Liverpool University Hospital Vertex Pharmaceuticals Incorporated UCB, Inc. Royal Hospital for Sick Children Trimeris Cystic Fibrosis Foundation Eisai Limited Eisai Inc. -

BIOCAT REPORT on the STATE of BIOTECHNOLOGY, BIOMEDICINE and MEDICAL TECHNOLOGY in CATALONIA © Biocat (Catalonia Bioregion Foundation)
BR09 BIOCAT REPORT ON THE STATE OF BIOTECHNOLOGY, BIOMEDICINE AND MEDICAL TECHNOLOGY IN CATALONIA © Biocat (Catalonia BioRegion Foundation) © for signed articles, the author BIOCAT Passeig Lluís Companys, 23 08010 Barcelona www.biocat.cat Authors: Nerea Alonso-Rodríguez, Laia Arnal, Manel Balcells, Josep Castells, Montse Daban, Adela Farré, Lluís Pareras, Marta Príncep, Pere Puigdomènech, Lluís Ruiz, Montserrat Vendrell. Coordination: Marta Príncep Published by: Biocat (Catalonia BioRegion Foundation) 1st edition: December 2009 Digital version. Available at: www.biocat.cat Design and layout: © Cromàtik Espai Creatiu This text may not be reproduced in whole or part without the express consent of the publisher (Biocat) and its authors. All rights to graphic design and artwork reserved. IB09 BIOCAT REPORT ON THE STATE OF BIOTECHNOLOGY, BIOMEDICINE AND MEDICAL TECHNOLOGY IN CATALONIA Table of contents Introduction 07 The importance of driving biotechnology as a countrywide strategy 09 Dr. Manel Balcells. President of the Biocat Executive Committee The strengths, weaknesses and challenges of a sector for the future 13 Dr. Montserrat Vendrell. CEO of Biocat Figures and scope 21 Trends and impact of biotechnology: opportunities for Catalonia 27 1. Biotechnology: trends and answers in a key sector of the economy 29 1.1. The biotech revolution 29 1.2. The biotech industry 29 1.3. The colors of biotech 32 1.4. Red biotech 33 1.5. Green biotech or agrifood: beyond GM foods 48 1.6. White biotech or industry: towards bioenergy 49 1.7. Biotechnology as a model for regional development: the cluster theory 50 2. Biotechnology applied to agriculture and food in Catalonia 53 Dr. -

UR-Coeus Sponsor List
UR-Coeus Sponsor Table (alphabetical by sponsor name) Sponsor Sponsor Code Sponsor Type 2138 21st Century Medicine CORP Sponsor Type Key 1 3M CORP CORP = Corporate 3803 4S3 Bioscience Inc CORP FED = Federal 5207 A. F. Associates Family Medicine ONFA FND = Foundation 2602 A. P. Pharma CORP IND = Individuals 2 A.L. Mailman Foundation FND NYS = New York State 2023 AA Implant Dentistry Research Foundation ONFA OLG = Other Local Gov't 3 AAA Fnd for Traffic Safety ONFA ONFA = Other Non-Fed Agency 2329 aaiPharma Inc CORP VHA = Voluntary Health Agency 3257 Aamjiwnaang First Nations Community ONFA 5345 Aarhus University Hospital ONFA 3713 Aaron Copland Fund for Music, Inc. ONFA 4 AARP Andrus Fdn FND 3853 AB Sciences CORP 3378 AB Vector, Inc CORP 899 Abbott Diagnostics CORP 3864 Abbott Fund FND 14 Abbott Laboratories CORP 5126 AbbVie, Inc. CORP 5854 Abeona Therapeutics CORP 5063 Abington Medical Specialists ONFA 5331 Abington Memorial Hospital ONFA 2835 ABIOMED, Inc. CORP 3687 Ablation Frontiers CORP 5118 Ablynx NV CORP 2053 ABMRF/Fnd for Alcohol Research ONFA 2171 Abortion Rights Mobilization ONFA 3361 Abraxix BioScience, LLC CORP 4953 Abt Associates, Inc. CORP 5037 Academic Gastrointestinal Cancer Consortium ONFA 15 Academic Med Ctr Cons ONFA 3733 Academic Pediatric Association ONFA 3160 Academy of Orofacial Pain ONFA 2122 Academy of Prosthodontics Foundation ONFA 3663 Acadia Pharmaceuticals, Inc. CORP 3273 Accelerate Brain Cancer Cure ONFA 5584 Acceleron Pharma Inc. CORP 5601 Accelovance, Inc. CORP 5940 Accreditation Council Grad Med Educ ONFA 5832 Accriva Diagnostics CORP 3888 AccuGenomics, Inc. CORP 5656 Acerta Pharma CORP 6101 Acessa Health, Inc. -

Financial Disclosures the Cophy EU Congress Seeks to Balance
Financial Disclosures The COPHy EU Congress seeks to balance the important benefits of physician-industry relationships with the potential risk that the financial goals of the industry may conflict with the professional goals of COPHy EU participants. In doing so, the COPHy EU Congress recognizes that it has a profound duty to its participants, the larger medical community and the public to ensure the integrity of all of its scientific, educational, and advocacy activities and materials. Educational Content Development The COPHy EU Congress requires all program members and any others in a position to control the educational content to disclose any commercial financial interests prior to the development of the educational content. A process of resolution of conflict of interest is used for those members who do have financial interests related to the development of the content. Presenters The COPHy EU Congress considers financial relationships to create actual conflicts of interest when Presenters or their immediate family (defined as spouse, domestic partner, parent, child or spouse of child, or sibling or spouse of sibling of the Presenter) have both a financial relationship with a commercial interest and the opportunity to affect the policy of the COPHy EU Congress of CME about the products or services of that commercial interest. The potential for Presenter to maintaining or increasing the value of the financial relationship with the commercial interest creates an incentive to influence the content of the CME – an incentive to insert commercial bias. All presenters of CME content should familiarize themselves with the policy of the COPHy EU Congress of CME based on the CME provider of the Congress – the EACCME (http://www.eaccme.AA/uemspdf/UEMS-2012-30.pdf ). -

FDA Workshop: Human Dermal Safety
Rethinking Human Dermal Safety Testing for Topical Drug Products Jonathan Wilkin, MD FDA Human Dermal Safety Testing Workshop September 10, 2018 Consulting Disclosures I • Aclaris • Adocia SA • Aisling Capital LLC • Aldeyra Therapeutic • Allergan Sales, LLC • Almirall SA • Amicus Therapeutics • Amryt Pharmaceuticals • Anacor Pharmaceuticals Consulting Disclosures II • Aponia Laboratories, Inc. • Applied Biology, Inc. • Arcutis, Inc. • Braintree Laboratories, Inc. • Brickell Biotech • CanFite • CapGenesis LLC • Capstone Development Services • Cara Therapeutics, Inc. Consulting Disclosures III • Cara Therapeutics, Inc. • Cassiopea S.p.A. • Castle Creek Pharmaceuticals, LLC • Chugai Pharma USA, Inc. • CinRx Pharma • Clarus Ventures, LLC • Chromaderm Inc • Clementia Pharmaceuticals Inc. • CODA Therapeutics, Inc. Consulting Disclosures IV • Corcept Therapeutics, Inc. • Cowen and Company • Crescita Therapeutics Inc. • Dalhousie University • Deb Group LTD • Demira Inc. • Dermex Pharmaceutical Inc. • Dermtreat ApS • DFB Soria, LLC Consulting Disclosures V • Dipexium Pharmaceuticals • Dr. Reddy’s Laboratories SA Swiss • Dr. Reddy’s Laboratories LTD (India) • DUSA Pharmaceuticals • Effexus Pharmaceutical, LLC • Ervin Epstein • Extera Partners • Foamix • Galderma Research & Development FR Consulting Disclosures VI • Genentech, Inc. • Genogen, Inc. • GlaxoSmithKline LLC • Genfit • Halozyme, Inc. • Hildred Capital Partners LLC • Histogen Inc. • Human Matrix Sciences, LLC • Incyte Corporation Consulting Disclosures VII • Immune Pharmaceuticals • Innovimmune -

Silence Therapeutics Initiation of Coverage
Silence Therapeutics Initiation of coverage Solving disease by targeting genes with siRNA Pharma & biotech 9 January 2020 We are initiating coverage of Silence Therapeutics (SLN), a developer of siRNA drugs for diseases that can be genetically targeted. Silence has a Price 334p proprietary platform for developing siRNA therapeutics, the strength of Market cap £262m which was highlighted by preclinical development deals with Mallinckrodt US$1.25/£ and Takeda (details below). The company will also be re-entering the clinic Net cash (£m) at 31 December 2019 33.5 in Q120 with SLN124, its own drug for iron overload. We are initiating with Shares in issue 78.4m a valuation of £345m or 440p per share. Free float 45.4% Code SLN Revenue PBT* EPS* DPS P/E Yield Year end (£m) (£m) (p) (p) (x) (%) Primary exchange AIM 12/17 0.0 (13.5) (7.7) 0.0 N/A N/A 12/18 0.0 (19.8) (25.2) 0.0 N/A N/A Secondary exchange OTCMKTS 12/19e 2.1 (18.2) (20.8) 0.0 N/A N/A Share price performance 12/20e 5.8 (22.1) (24.2) 0.0 N/A N/A Note: *PBT and EPS are normalised, excluding amortisation of acquired intangibles, exceptional items and share-based payments. Takeda and Mallinckrodt deals endorse platform Silence announced in July 2019 that it signed a deal with Mallinckrodt for the rights to an undisclosed C3 complement inhibitor, SLN500. The deal included $20m upfront, up to $673m in milestone payments (of which $2m has been delivered), double-digit royalties and options on two other complement assets. -

Orphan Drug Designations and Approvals List As of 12-02-2013 Governs January 1, 2014
Orphan Drug Designations and Approvals List as of 12‐02‐2013 Governs January 1, 2014 ‐ March 31, 2014 Row Designation Generic Name Trade Name Orphan Designation Contact Company/Sponsor Num Date Treatment of Follicular Lymphoma, Small Lymphocytic Lymphoma, Lymphoplasmacytic Lymphoma, Splenic Marginal Zone Lymphoma, Extranodal Marginal Zone B‐cell Lymphoma of Mucosa‐ Associated Lymphoma Tissue (MALT), and Nodal Marginal Zone Lymphoma (Collectively Indolent B‐cell Non‐Hodgkin's 1 bendamustine hydrochloride Treanda 11/26/2013 Lymphoma) Cephalon, Inc. Treatment of subjects at risk of developing myelosuppression after a radiological or nuclear 2 Filgrastim Neupogen 11/20/2013 incident Amgen, Inc. adeno‐associated viral vector containing the human NADH Treatment of Leber Hereditary 3 Dehydrogenase 4 Gene n/a 11/20/2013 Optic Neuropathy Gen Sight Biologics Treatment of liver transplant recipients with reestablished fibrosis to delay the progression to cirrhosis and 4 emricasan n/a 11/20/2013 end stage liver disease Conatus Pharmaceuticals Inc. Page 1 of 262 Orphan Drug Designations and Approvals List as of 12‐02‐2013 Governs January 1, 2014 ‐ March 31, 2014 5‐((4‐bromo‐2‐ fluorophenyl)amino)‐4‐fluoro‐N‐(2‐ hydroxyethoxy)‐1‐methyl‐1H‐ Treatment Stage IIB‐IV Novartis Pharmaceuticals 5 benzo[d]imidazole‐6‐carboxamide n/a 11/19/2013 melanoma. Corporation Treatment of Hepatitis Delta 6 Lonafarnib n/a 11/19/2013 Virus (HDV)infection Eiger Biopharmaceuticals, Inc. Treatment in Stage IIB‐IV melanoma positive for the Novartis Pharmaceuticalues 7 MEK162 -
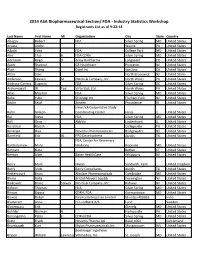
FDA - Industry Statistics Workshop Registrants List As of 9-22-14
2014 ASA Biopharmaceutical Section/ FDA - Industry Statistics Workshop Registrants List as of 9-22-14 Last Name First Name MI Organization City State Country Abugov Robert FDA Silver Spring MD United States Acusta Andre Wayne PA United States Adachi Yoko FDA College Park MD United States Ahn Chul H. FDA-CDRH Silver Spring MD United States Aitchison Roger D. Array BioPharma Longmont CO United States Alam Shamsul GE Healthcare Princeton NJ United States Alavi Shariq Cytel Inc. San Jose CA United States Altan Stan North Brunswick NJ United States Anderson Keaven M. Merck & Company, Inc. North Wales PA United States Andraca-Carrera Eugenio FDA Silver Spring DC United States Archambault W. Tad Virtu Stat, Ltd. North Wales PA United States Atlas Mourad FDA Silver Spring MD United States Baba Yuko Shionogi Inc. Florham Park NJ United States Badre Sejal Amgen Providence RI United States Hines VA Cooperative Study Bahn Gideon D. Coordinating Center Hines IL United States Bai Steve FDA Silver Spring MD United States Ball Greg AbbVie Lindenhurst IL United States Bandekar Rajesh Collegeville PA United States Banerjee Hiya Novartis Pharmaceuticals Bridgewater NJ United States Barefield Eric W. PPD Development Austin TX United States FDA, Center for Veterinary Bartholomew Mary J. Medicine Rockville MD United States Bennett Nate Bethel CT United States Benson Alice Bayer HealthCare Whippany NJ United States Berry Mark Mylan Sandwich, Kent United Kingdom Berry Scott Berry Consultants Austin TX United States Bettencourt Brian Alnylam Pharmaceuticals Cambridge MA United States Bhore Rafia Bristol-Meyers Squibb Pennington NJ United States Binkowitz Bruce Steven Merck & Company, Inc. Rahway NJ United States Birkner Thomas FDA Silver Spring MD United States Biswas Bipasa CDRH, FDA Germantown MD United States Biswas Debjit Piramal Enterprises Limited Mumbai 400063 India Bladstrom Anna H.