Stage New Drug Discovery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2009: Turning the Corner
data page 2009: Turning the corner Walter Yang Despite the shaky start to 2009, the biotech sector regained its financial Although initial public offerings showed signs of resuscitation (at least footing. Biotech indices were up, as were offerings and partnership mon- 13 more companies are now in the queue), follow-on financings came in ies. Excluding collaborations, the sector raised a total of $24.3 billion. above $6 billion—the second-best year over the past decade. Stock market performance Global biotech industry financing Although biotech indices were up ~16% last year, they underperformed The boost in partnership promises to US biotechs and follow-on financings other major indices. pushed industry funding to $61.3 billion, up 82% from 2008. 1,500 Swiss Market S&P 500 2009 36.9 10.0 5.1 2.2 6.0 0.9 1,400 NASDAQ Biotech Dow Jones 2008 20.0 3.2 5.3 3.1 1.9 0.1 1,300 NASDAQ BioCentury 100 2007 22.4 11.7 6.8 4.7 4.4 3.0 1,200 Partnering 2006 19.8 11.9 5.6 4.7 5.6 2.0 1,100 Year Debt and other Index 17.3 6.1 5.4 2.7 4.8 1.9 1,000 2005 Venture capital PIPEs 900 2004 10.9 8.8 5.3 2.9 3.3 2.6 Follow-ons 800 2003 8.9 9.1 4.0 2.2 3.9 0.5 IPOs 700 010203040506070 Amount raised ($ billions) 1/09 2/09 3/09 4/09 5/09 6/09 7/09 8/09 9/09 12/08 Month ending 10/09 11/09 12/09 Partnership figures are for deals involving a US company. -
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
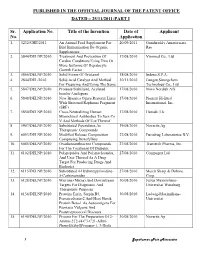
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

Infectious Diseases
2013 MEDICINES IN DEVELOPMENT REPORT Infectious Diseases A Report on Diseases Caused by Bacteria, Viruses, Fungi and Parasites PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Evolves Against Infectious Diseases with Nearly 400 Medicines and Vaccines in Testing Throughout history, infectious diseases hepatitis C that inhibits the enzyme have taken a devastating toll on the lives essential for viral replication. and well-being of people around the • An anti-malarial drug that has shown Medicines in Development world. Caused when pathogens such activity against Plasmodium falci- For Infectious Diseases as bacteria or viruses enter a body and parum malaria which is resistant to multiply, infectious diseases were the current treatments. Application leading cause of death in the United Submitted States until the 1920s. Today, vaccines • A potential new antibiotic to treat methicillin-resistant Staphylococcus Phase III and infectious disease treatments have proven to be effective treatments in aureus (MRSA). Phase II many cases, but infectious diseases still • A novel treatment that works by Phase I pose a very serious threat to patients. blocking the ability of the smallpox Recently, some infectious pathogens, virus to spread to other cells, thus 226 such as pseudomonas bacteria, have preventing it from causing disease. become resistant to available treatments. Infectious diseases may never be fully Diseases once considered conquered, eradicated. However, new knowledge, such as tuberculosis, have reemerged new technologies, and the continuing as a growing health threat. commitment of America’s biopharma- America’s biopharmaceutical research ceutical research companies can help companies are developing 394 medicines meet the continuing—and ever-changing and vaccines to combat the many threats —threat from infectious diseases. -

178S ASMS Directory of Members DAVID AASERUD the Lubrizol
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector 178S ASMS Directory of Members DAVID AASERUD SUZANNE ACKLOO WILLIAM ADAMS The Lubrizol Corporation MDS Sciex Philip Morris USA 29400 Lakeland Blvd. 71 Four Valley Drive RD&E/OC-T3W Wickliffe, OH 44092 Concord, ON L4K 4V8 Canada 615 Maury Street Tel: 440 347 4776 Tel: 905 660 9005 Richmond, VA 23224 [email protected] [email protected] Tel: 804 274 2093 [email protected] SUSAN ABBATIELLO EUREKA ACOLATSE University of Florida 7237 Causeway Dr. #3B GARY E. ADAMSON Department of Chemistry Indianapolis, IN 46214 Merck and Co. PO Box 117200 Tel: 317 433 4016 Merch Research Laboratories Gainesville, FL 32611 [email protected] P.O. Box 4 Tel: 352 392 0536 West Point, PA 19486 [email protected] CHRIS ADAMS Tel: 215 652 1174 Uppsala University [email protected] LARRY ABBEY Biological & Medical Mass Spec Waters Corporation Box 583, BMC JULIE ADAMSON 4026 Oak Crest Drive Uppsala, SE-751 23 Sweden University of Michigan Tucker, GA 30084 Tel: 46 18 471 5729 930 N. University Tel: 770 414 5089 [email protected] Ann Arbor, MI 48109-1055 [email protected] Tel: 734-763-6535 GREG ADAMS [email protected] FRANK S. ABBOTT Diosynth Biotechnology University of British Columbia 3000 Weston Parkway TOM ADDISON Faculty of Pharmaceutical Science Cary, NC 27513 Covance-11 2146 East Mall Tel: 919 388 5690 6002/11 Vancouver, BC V6T 1Z3 Canada [email protected] 3301 Kinsman Boulevard Tel: 604 822 2566 Madison, WI 53704-2523 [email protected] LUKE ADAMS Tel: 608 242 2639 University of Washington, Chemistry [email protected] FADI ABDI Box 351700 Applied Biosystems Seattle, WA 98195 TERRI ADDONA 500 Old Connecticut Path Tel: 206 543 7656 Broad Instritute Framingham, MA 01702 [email protected] 320 Charles Street Tel: 508 383 7921 Cambridge, MA 02141 [email protected] NIGEL G. -

RSAP1359 Proof 41..55
MEDICINES AUTHORITY REGULATOR AND INDUSTRY PERCEPTIONS OF BIOSIMILAR INTERCHANGEABILITY Druedahl, Louise C.; De Bruin, Marie L.; Hoogland, Hans; Minssen, Timo; van de Weert, Marco; Sporrong, Sofia Kalvemark; Almarsdottir, Anna Birna DOI: 10.1016/j.sapharm.2019.09.006 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Druedahl, L. C., De Bruin, M. L., Hoogland, H., Minssen, T., van de Weert, M., Sporrong, S. K., & Almarsdottir, A. B. (2019). MEDICINES AUTHORITY REGULATOR AND INDUSTRY PERCEPTIONS OF BIOSIMILAR INTERCHANGEABILITY. E45-E46. https://doi.org/10.1016/j.sapharm.2019.09.006 Download date: 28. sep.. 2021 Research in Social and Administrative Pharmacy 15 (2019) e41ee55 Contents lists available at ScienceDirect Research in Social and Administrative Pharmacy journal homepage: www.rsap.org 9th Nordic Social Pharmacy Conference 2019. Conference abstracts ATTITUDES TOWARDS DEPRESCRIBING IN OLDER ADULTS WITH Pottegård a,c. a Hospital Pharmacy Funen, Odense University Hospital, LIMITED LIFE EXPECTANCY: TWO SYSTEMATIC REVIEWS Odense, Denmark; b OPEN, Odense Patient data Explorative Network, Odense, Denmark; c Clinical Pharmacology and Pharmacy, Department of Alaa Burghle a,b, Carina Lundby a,b, Jesper Ryg c,d, Jens Søndergaard e, Anton Public Health, University of Southern Denmark, Odense, Denmark; Pottegård a,b, Trine Graabæk a,b, Dorthe Nielsen f,g,h. a Hospital Pharmacy d Research and Development, Danish College of Pharmacy Practice, Hillerød, Funen, Odense University Hospital, Odense C, Denmark; b Clinical Denmark; e Social and Clinical Pharmacy, School of Pharmacy, Copenhagen Pharmacology and Pharmacy, Department of Public Health, University of University, Copenhagen, Denmark Southern Denmark, Odense C, Denmark; c Department of Geriatric E-mail address: [email protected] (A. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -
Annex 2: Providers Required to Respond (Red Indicates Those Who Did Not Respond Within the Required Timeframe)
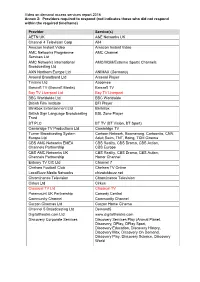
Video on demand access services report 2016 Annex 2: Providers required to respond (red indicates those who did not respond within the required timeframe) Provider Service(s) AETN UK A&E Networks UK Channel 4 Television Corp All4 Amazon Instant Video Amazon Instant Video AMC Networks Programme AMC Channel Services Ltd AMC Networks International AMC/MGM/Extreme Sports Channels Broadcasting Ltd AXN Northern Europe Ltd ANIMAX (Germany) Arsenal Broadband Ltd Arsenal Player Tinizine Ltd Azoomee Barcroft TV (Barcroft Media) Barcroft TV Bay TV Liverpool Ltd Bay TV Liverpool BBC Worldwide Ltd BBC Worldwide British Film Institute BFI Player Blinkbox Entertainment Ltd BlinkBox British Sign Language Broadcasting BSL Zone Player Trust BT PLC BT TV (BT Vision, BT Sport) Cambridge TV Productions Ltd Cambridge TV Turner Broadcasting System Cartoon Network, Boomerang, Cartoonito, CNN, Europe Ltd Adult Swim, TNT, Boing, TCM Cinema CBS AMC Networks EMEA CBS Reality, CBS Drama, CBS Action, Channels Partnership CBS Europe CBS AMC Networks UK CBS Reality, CBS Drama, CBS Action, Channels Partnership Horror Channel Estuary TV CIC Ltd Channel 7 Chelsea Football Club Chelsea TV Online LocalBuzz Media Networks chizwickbuzz.net Chrominance Television Chrominance Television Cirkus Ltd Cirkus Classical TV Ltd Classical TV Paramount UK Partnership Comedy Central Community Channel Community Channel Curzon Cinemas Ltd Curzon Home Cinema Channel 5 Broadcasting Ltd Demand5 Digitaltheatre.com Ltd www.digitaltheatre.com Discovery Corporate Services Discovery Services Play -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

Configuration Manager User Guide © 2019 Quest Software Inc
Quest® Enterprise Reporter 3.2.1 Configuration Manager User Guide © 2019 Quest Software Inc. ALL RIGHTS RESERVED. This guide contains proprietary information protected by copyright. The software described in this guide is furnished under a software license or nondisclosure agreement. This software may be used or copied only in accordance with the terms of the applicable agreement. No part of this guide may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying and recording for any purpose other than the purchaser’s personal use without the written permission of Quest Software Inc. The information in this document is provided in connection with Quest Software products. No license, express or implied, by estoppel or otherwise, to any intellectual property right is granted by this document or in connection with the sale of Quest Software products. EXCEPT AS SET FORTH IN THE TERMS AND CONDITIONS AS SPECIFIED IN THE LICENSE AGREEMENT FOR THIS PRODUCT, QUEST SOFTWARE ASSUMES NO LIABILITY WHATSOEVER AND DISCLAIMS ANY EXPRESS, IMPLIED OR STATUTORY WARRANTY RELATING TO ITS PRODUCTS INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. IN NO EVENT SHALL QUEST SOFTWARE BE LIABLE FOR ANY DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE, SPECIAL OR INCIDENTAL DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS, BUSINESS INTERRUPTION OR LOSS OF INFORMATION) ARISING OUT OF THE USE OR INABILITY TO USE THIS DOCUMENT, EVEN IF QUEST SOFTWARE HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. Quest Software makes no representations or warranties with respect to the accuracy or completeness of the contents of this document and reserves the right to make changes to specifications and product descriptions at any time without notice. -

Pfizer Inr 235 East 42Nd Street New York
Pfizer Inr 235 East 42nd Street New York. !W 10007-5755 March 25,2008 Steven Reynolds Director U.S. Nuclear Regulatory Commission Division of Nuclear Materials Safety 2443 Warrenville Road, STE 210 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42"d Street in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. panof the Board C ief Executive Officer Pfizer lnr 235 East 42nd Street New York, NY 10007-5755 March 25. 2008 Steven Reynolds Director U.S. Nuclear Regulatory Coinmission Division of Nuclear Material Safet) 2443 Warrenville Road STE 2 IO Lisle. IL 60532-4352 -Re: Financial Assurance Demonstration for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the chief financial officer of Pfizer Inc., 235 East 42'ld Street, New York. New York 10017, a corporation. This letter is in support of this firm's use of the parent company guarantee financial test to demonstrate financial assurance, as specified in IO CFR Part 30. -
Channel Guide August 2018
CHANNEL GUIDE AUGUST 2018 KEY HOW TO FIND WHICH CHANNELS YOU HAVE 1 PLAYER PREMIUM CHANNELS 1. Match your ENTERTAINMENT package 1 2 3 4 5 6 2 MORE to the column 100 Virgin Media Previews 3 M+ 101 BBC One If there’s a tick 4 MIX 2. 102 BBC Two in your column, 103 ITV 5 FUN you get that 104 Channel 4 6 FULL HOUSE channel ENTERTAINMENT SPORT 1 2 3 4 5 6 1 2 3 4 5 6 100 Virgin Media Previews 501 Sky Sports Main Event 101 BBC One HD 102 BBC Two 502 Sky Sports Premier 103 ITV League HD 104 Channel 4 503 Sky Sports Football HD 105 Channel 5 504 Sky Sports Cricket HD 106 E4 505 Sky Sports Golf HD 107 BBC Four 506 Sky Sports F1® HD 108 BBC One HD 507 Sky Sports Action HD 109 Sky One HD 508 Sky Sports Arena HD 110 Sky One 509 Sky Sports News HD 111 Sky Living HD 510 Sky Sports Mix HD 112 Sky Living 511 Sky Sports Main Event 113 ITV HD 512 Sky Sports Premier 114 ITV +1 League 115 ITV2 513 Sky Sports Football 116 ITV2 +1 514 Sky Sports Cricket 117 ITV3 515 Sky Sports Golf 118 ITV4 516 Sky Sports F1® 119 ITVBe 517 Sky Sports Action 120 ITVBe +1 518 Sky Sports Arena 121 Sky Two 519 Sky Sports News 122 Sky Arts 520 Sky Sports Mix 123 Pick 521 Eurosport 1 HD 132 Comedy Central 522 Eurosport 2 HD 133 Comedy Central +1 523 Eurosport 1 134 MTV 524 Eurosport 2 135 SYFY 526 MUTV 136 SYFY +1 527 BT Sport 1 HD 137 Universal TV 528 BT Sport 2 HD 138 Universal -

The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry
The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry By: Maitri Punjabi Honors Thesis Economics Department The University of North Carolina at Chapel Hill March 2016 Approved: ______________________________ Dr. Jonathan Williams Punjabi 2 Abstract This paper analyzes the effect of the changing nature of innovation on pharmaceutical firm market value from the years 1987 to 2010 by using U.S. patent and claim data. Over the years, firms have started shifting focus from primary innovation to secondary innovation as new ideas and new compounds become more difficult to generate. In this study, we analyze the impact of this patent portfolio shift on the market capitalization of pharmaceutical firms. After using firm fixed effects and the instrumental variable approach, we find that there exists a strong positive relationship between secondary innovations and the market value of the firm– in fact, we find a stronger relationship than is observed between primary innovation and market value. When focusing on the different levels of innovation within the industry, we find that this relationship is stronger for less-innovative firms (those that have produced fewer patents) than it is for highly- innovative firms. We also find that this relationship is stronger for firms that spend less on research and development, complementing earlier findings that research productivity is declining over time. Punjabi 3 Acknowledgements I would primarily like to thank my adviser, Dr. Jonathan Williams, for his patience and constant support. Without his kind and helpful attitude, this project would have been a much more frustrating process. Through his knowledge of the industry, I have gained valuable insight and have learned a great deal about a unique and growing field.