EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
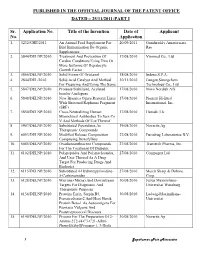
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

Kopi Af Aktivlisten 2021-06-30 Ny.Xlsm
Velliv noterede aktier i alt pr. 30-06-2021 ISIN Udstedelsesland Navn Markedsværdi (i DKK) US0378331005 US APPLE INC 1.677.392.695 US5949181045 US MICROSOFT CORP 1.463.792.732 US0231351067 US AMAZON.COM INC 1.383.643.996 DK0060534915 DK NOVO NORDISK A/S-B 1.195.448.146 US30303M1027 US FACEBOOK INC-CLASS A 1.169.094.867 US02079K3059 US ALPHABET INC-CL A 867.740.769 DK0010274414 DK DANSKE BANK A/S 761.684.457 DK0060079531 DK DSV PANALPINA A/S 629.313.827 US02079K1079 US ALPHABET INC-CL C 589.305.120 US90138F1021 US TWILIO INC - A 514.807.852 US57636Q1040 US MASTERCARD INC - A 490.766.560 US4781601046 US JOHNSON & JOHNSON 478.682.981 US70450Y1038 US PAYPAL HOLDINGS INC 471.592.728 DK0061539921 DK VESTAS WIND SYSTEMS A/S 441.187.698 US79466L3024 US SALESFORCE.COM INC 439.114.061 US01609W1027 US ALIBABA GROUP HOLDING-SP ADR 432.325.255 US8835561023 US THERMO FISHER SCIENTIFIC INC 430.036.612 US22788C1053 US CROWDSTRIKE HOLDINGS INC - A 400.408.622 KYG875721634 HK TENCENT HOLDINGS LTD 397.054.685 KR7005930003 KR SAMSUNG ELECTRONICS CO LTD 389.413.700 DK0060094928 DK ORSTED A/S 378.578.374 ES0109067019 ES AMADEUS IT GROUP SA 375.824.429 US46625H1005 US JPMORGAN CHASE & CO 375.282.618 US67066G1040 US NVIDIA CORP 357.034.119 US17275R1023 US CISCO SYSTEMS INC 348.160.692 DK0010244508 DK AP MOLLER-MAERSK A/S-B 339.783.859 US20030N1019 US COMCAST CORP-CLASS A 337.806.502 NL0010273215 NL ASML HOLDING NV 334.040.559 CH0012032048 CH ROCHE HOLDING AG-GENUSSCHEIN 325.008.200 KYG970081173 HK WUXI BIOLOGICS CAYMAN INC 321.300.236 US4370761029 US HOME DEPOT INC 317.083.124 US58933Y1055 US MERCK & CO. -

IQVIA Pharma Deals Half-Year Review of 2020
White Paper IQVIA Pharma Deals Half-Year Review of 2020 HEATHER CARTWRIGHT, Senior Analyst, Global Market Insights, IQVIA MICHELLE LIU, Analyst, Global Market Insights, IQVIA TASKIN AHMED, Manager, Global Market Insights, IQVIA Table of contents Introduction 1 Uncertainty and prudence cause M&A to stall 2 Licensing deal values continue to rise 5 Roche pips AstraZeneca to title of most active dealmaker 10 COVID-19 pandemic drives increase in R&D collaboration 11 Infectious diseases replace oncology as top therapeutic area for dealmaking 14 Outlook for H2 2020 15 About the authors 17 Introduction COVID-19 shakes up the dealmaking landscape H1 2020 was an unprecedented time for dealmaking in the life sciences sector. While the COVID-19 pandemic rapidly provoked a significant level of partnering activity amongst biopharma companies keen to expedite the development of vaccines or therapeutics to tackle the virus, it also acted as a brake on other types of dealmaking, most notably M&A which saw a sharp decline in activity as a result of uncertainty and operational disruption. Indeed, H1 2020 was notable for the absence of any US$5 B deals on the scale seen in previous years as companies instead focused their attentions on internal programs and potential COVID-19 solutions, opting only for a few asset-driven acquisitions. There was also plentiful capital available to allow biotech companies to stay independent, particularly those in the US. As a result, aggregate spending on M&A by life science companies plummeted to just US$19.1 B in the first 6 months of 2020. -

Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Reduction in the Risk of Major Adverse Cardiovascular Events
Reduction in the Risk of Major Adverse Cardiovascular Events with Apabetalone, a BET Protein Inhibitor, in Patients with Recent Acute Coronary Syndrome and Type 2 Diabetes According to Insulin Treatment: Analysis of the BETonMACE Trial Gregory G. Schwartz1, Stephen J. Nicholls2, Henry N. Ginsberg3, Jan O. Johansson4, Kamyar Kalantar‐Zadeh5, Ewelina Kulikowski4, Peter P Toth6, Norman Wong4, Michael Sweeney4, Kausik K Ray7 On behalf of the BETonMACE Investigators 1Division of Cardiology, University of Colorado School of Medicine, Aurora, CO USA; 2Monash University Cardiovascular Research Center, Melbourne, Australia; 3Vagelos College of Physicians and Surgeons, Columbia University, New York, NY USA; 4Resverlogix Corp., Calgary, Alberta, Canada; 5University of California Irvine School of Medicine, Orange, CA USA; 6CGH Medical Center, Sterling, IL and Johns Hopkins University, Baltimore, MD USA; 7Imperial College Center for Cardiovascular Disease Prevention, London UK American Heart Association – 2020 Scientific Sessions November 2020 ClinicalTrials.gov: NCT02586155 Disclosures • The BETonMACE trial was funded by Resverlogix • Dr Schwartz reports research grants to the University of Colorado from Resverlogix, Sanofi, The Medicines Company, and Roche; and is coinventor of pending US patent 14/657192 (“Methods of Reducing Cardiovascular Risk”) assigned in full to the University of Colorado. • Dr. Nicholls reports grants from Resverlogix, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from -

Unlocking the Potential of Gene Silencing for Everyone
Corporate Presentation JP Morgan Conference January 2018 Forward looking statements The information contained in this presentation is being supplied and communicated to you on a Securities in the Company have not been, and will not be, registered under the United States confidential basis solely for your information and may not be reproduced, further distributed to Securities Act of 1933, as amended (the “Securities Act”), or qualified for sale under the law of any other person or published, in whole or in part, for any purpose. In accordance with the any state or other jurisdiction of the United States of America and may not be offered or sold in prohibition on market abuse contained in Part VIII of the Financial Services and Markets Act the United States of America except pursuant to an exemption from, or in a transaction not 2000 (as amended) (the “Act”): (i) you must not pass this information to any person; and (ii) subject to, the registration requirements of the Securities Act. Neither the United States you must not base any behaviour in relation to any securities or other Qualifying Investments Securities and Exchange Commission nor any securities regulatory body of any state or other (as that term is defined in the Act) which would amount to market abuse on such information jurisdiction of the United States of America, nor any securities regulatory body of any other until after it is made generally available. country or political subdivision thereof, has approved or disapproved of this presentation or the securities discussed herein or passed on the accuracy or adequacy of the contents of this This presentation is being communicated in the United Kingdom only to (a) persons who have presentation. -

GSK's Big Reveal
3 August 2018 No. 3916 Scripscrip.pharmaintelligence.informa.com Pharma intelligence | informa at Genentech Inc. (Also see “GSK Bags Bar- ron As R&D Boss As Vallance Joins UK Govern- ment” - Scrip, 8 Nov, 2017.) With a budding early immuno-oncology pipeline, Walmsley clearly has her eye on establishing GSK as an important player in the space. The event in London was Barron’s first op- portunity to present his R&D strategy and coincided with the company’s second quar- ter sales and earnings release. “This is an ideal time to be thinking about reinventing R&D, because we are doing well. We are growing,” Barron said. PILLARS FOR INNOVATION Investors have been eager to hear from the R&D legend, but with few near-term catalysts or big changes to the late-stage pipeline, they may be underwhelmed by the update. Barron, meanwhile, is focused on deliver- GSK’s Big Reveal: ing scientific and cultural changes that will yield breakthroughs over the long-term. An R&D Overhaul Poised To Yield He talked about a time horizon of 2021 to 2026, and he talked more high-level, rather than specifics, about incentives needed to Long-Term Cultural Change drive innovation, the technologies that can JESSICA MERRILL [email protected] help improve clinical trial success, and cul- tural changes. He said science, technology laxoSmithKline PLC CEO Emma pharma R&D – is that investors will need to and culture are the three pillars needed to Walmsley may have spelled out the be patient to see GSK deliver on its promise drive innovation, and all three must be in Gbig pharma’s R&D turnaround plan of innovation. -

Recent Advances in Oligonucleotide Therapeutics in Oncology
International Journal of Molecular Sciences Review Recent Advances in Oligonucleotide Therapeutics in Oncology Haoyu Xiong 1, Rakesh N. Veedu 2,3 and Sarah D. Diermeier 1,* 1 Department of Biochemistry, University of Otago, Dunedin 9016, New Zealand; [email protected] 2 Centre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth 6150, Australia; [email protected] 3 Perron Institute for Neurological and Translational Science, Perth 6009, Australia * Correspondence: [email protected] Abstract: Cancer is one of the leading causes of death worldwide. Conventional therapies, including surgery, radiation, and chemotherapy have achieved increased survival rates for many types of cancer over the past decades. However, cancer recurrence and/or metastasis to distant organs remain major challenges, resulting in a large, unmet clinical need. Oligonucleotide therapeutics, which include antisense oligonucleotides, small interfering RNAs, and aptamers, show promising clinical outcomes for disease indications such as Duchenne muscular dystrophy, familial amyloid neuropathies, and macular degeneration. While no approved oligonucleotide drug currently exists for any type of cancer, results obtained in preclinical studies and clinical trials are encouraging. Here, we provide an overview of recent developments in the field of oligonucleotide therapeutics in oncology, review current clinical trials, and discuss associated challenges. Keywords: antisense oligonucleotides; siRNA; aptamers; DNAzymes; cancers Citation: Xiong, H.; Veedu, R.N.; 1. Introduction Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in According to the Global Cancer Statistics 2018, there were more than 18 million new Oncology. Int. J. Mol. Sci. 2021, 22, cancer cases and 9.6 million deaths caused by cancer in 2018 [1]. -

יומן הפטנטים והמדגמים Patents and Designs Journal
י /' התשס"ח 5/2008 רשומות ISRAEL STATE RECORDS ו' באב התשס"ח August 7, 2008 יומן הפטנטים והמדגמים PATENTS AND DESIGNS JOURNAL פטנטים עמוד PATENTS Page בקשות שהוגשו Applications filed 1507 בקשות שקובלו Applications accepted 1775 פטנטים שניתנו Patents granted 1992 פטנטים שחודשו Patents renewed 1993 פטנטים שתוקפם פקעו Patents not in force 1995 פטנטים שחודשו לעשרים שנה Patents renewed for 20 years 1996 פטנטים שפג תוקפם Patents expired 1997 הודעות Notices 1998 שינויים בפרטים רשומים Changes in particulars entered בפנקס in register 2001 תיקוני טעויות Corrigenda 2002 מפתחות לבקשות שקובלו Indices of applications accepted i מדגמים DESIGNS מדגמים שנרשמו Designs registered 2004 מדגמים שחודשו Designs renewed 2017 מדגמים שבוטלו Designs void 2018 ו' באב התשס"ח – August 7, 2008 1507 ידיעות כלליות מכתבים, מסמכים, וכו' בענייני פטנטים ומדגמים יש לשלוח אל: רשם הפטנטים והמדגמים, רח' הסדנא 4, ירושלים לשכת הפטנטים נמצאת ברח' הסדנא 4, תלפיות, ירושלים והיא פתוחה לציבור בימי חול שאינם ערבי שבת או מועד בין השעות 8:30 ו - 12:30. לשכת הפטנטים מספקת תצלומים של פירוטים ושרטוטים במחיר של 2.50 שקלים בעד כל עמוד או חלק ממנו. אגרות ללשכת הפטנטים מתקבלות אך ורק על ידי תשלום לחשבון הלשכה בבנק הדואר מס' 0-24145-2. יש להציג קבלת בנק הדואר ללשכה יחד עם הבקשה לפעולה שעבורה האגרה שולמה. GENERAL INFORMATION Letters, documents, etc. concerning Patents and Designs should be addressed to: The Commissioner of Patents and Designs, 4 Hasadnah St., Jerusalem The Patent Office is located at 4 Hasadnah St., Talpiot, Jerusalem and is open to the public on weekdays, except on Fridays or on the eves of holydays, from 08:30 to 12:30 hrs. -

Combining High-Sensitivity Troponin with the AHA/ACC Cholesterol
Combining High -Sensitivity Troponin with the AHA/ACC Cholesterol Guidelines To Guide Evolocumab Therapy Nicholas A. Marston, MD, MPH, Kazuma Oyama, MD, PhD, Petr Jarolim, MD, PhD, Minao Tang, MS, Peter S. Sever, PhD, FRCP, Anthony C. Keech, MD, Armando Lira Pineda, MD, Huei Wang, PhD, Robert P. Giugliano, MD, SM, Marc S. Sabatine, MD, MPH, David A. Morrow, MD, MPH Insights into Plaque Vulnerability, Stabilization and Mechanisms of Death in Stable Coronary Artery Disease Friday, November 13th, 2020 #AHA20 Disclosures Presenter: clinical trial involvement with Amgen, Pfizer, Novartis, and AstraZeneca without personal fees, payments, or salary increase. Co-Authors: KO reports grant support from JSPS Overseas Research Fellowships. PJ reports research support from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi-Sankyo, Inc., Eisai, Inc., GlaxoSmithKline, Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Roche Diagnostics Corporation, Siemens Healthineers, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics Corporation. MT reports no disclosures. PS reports research grants and honoraria for speakers bureau- Amgen and Pfizer. ACK reports grants and personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Mylan, personal fees from Pfizer, grants from Sanofi, grants from Novartis, personal fees from Bayer, outside the submitted work. ALP was previously employed by Amgen, now employed by Arrowhead Pharmaceutical. -

2019 Annual Meeting Education Committee All Relationships Are Considered Compensated
2019 Annual Meeting Education Committee All relationships are considered compensated. Relationships are self-held unless otherwise noted. I = Immediate Family Member, Inst = My Institution Patents, Royalties, Travel, Stock and Other Expert Name Employment Leadership Honoraria Consulting or Advisory Role Speakers' Bureau Research Funding Other Intellectual Accommodations, Other Ownership Interests Testimony Property Expenses Monica M. Abbvie (Inst), Agenus (Inst), Astellas Bertagnolli Pharma (Inst), AstraZeneca (Inst), Baxalta (Inst), Bayer Health (Inst), Breast Cancer Research Foundation (Inst), Bristol- Myers Squibb (Inst), Celgene (Inst), Complion (Inst), Eisai (Inst), Exelixis (Inst), Genentech (Inst), GHI Pharma (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Janssen (Inst), Jazz Pharmaceuticals (Inst), Leidos (Inst), Lexicon (Inst), Lilly (Inst), Matrex (Inst), Mayo Clinic (Inst), Merck (Inst), MGH (Inst), Millenium Pharamceuticals (Inst), Novartis (Inst), PCORI (Inst), Pfizer (Inst), Pharmacyclics (Inst), Robert Wood Johnson Foundation (Inst), Sagerock Advisors (Inst), Sanofi (Inst), Taiho Oncology (Inst), Takeda (Inst), Tesaro (Inst), Teva (Inst) Tatiana M. Prowell David R. Spigel AstraZeneca (Inst), Boehringer AstraZeneca (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Ingelheim (Inst), Bristol-Myers Squibb Squibb (Inst), Celgene (Inst), (Inst), Celgene (Inst), Genentech/Roche Genentech/Roche (Inst), (Inst), Lilly (Inst), Merck (Inst), Novartis Novartis (Inst), Pfizer (Inst) (Inst), Pfizer (Inst), University -

SILENCE THERAPEUTICS PLC (Exact Name of Registrant As Specified in Its Charter)
Table of Contents As filed with the Securities and Exchange Commission on April 8, 2021 Registration No. 333-248203 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Post-Effective Amendment No. 2 to Form F-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 SILENCE THERAPEUTICS PLC (Exact Name of Registrant as Specified in its Charter) Not Applicable (Translation of Registrant’s Name into English) England and Wales 2834 Not Applicable (State or other Jurisdiction of (Primary Standard Industrial (I.R.S. Employer Incorporation or Organization) Classification Code Number) Identification Number) 72 Hammersmith Road London W14 8TH United Kingdom Tel: +44 20 3457 6900 (Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) Silence Therapeutics Inc. 434 West 33rd Street, Office 814 New York, New York 10001 Tel: +1 917 374 0372 (Name, address, including zip code, and telephone number, including area code, of agent for service) Copies of all communications, including communications sent to agent for service, should be sent to: Joshua A. Kaufman Claire A. Keast-Butler Divakar Gupta Cooley (UK) LLP Brian F. Leaf Dashwood Cooley LLP 69 Old Broad Street 55 Hudson Yards London EC2M 1QS New York, New York 10001 United Kingdom +1 212 479—6000 +44 20 7785 9355 Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.