יומן הפטנטים והמדגמים Patents and Designs Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
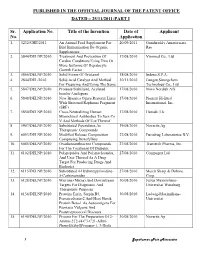
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

Pfizer Inr 235 East 42Nd Street New York
Pfizer Inr 235 East 42nd Street New York. !W 10007-5755 March 25,2008 Steven Reynolds Director U.S. Nuclear Regulatory Commission Division of Nuclear Materials Safety 2443 Warrenville Road, STE 210 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42"d Street in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. panof the Board C ief Executive Officer Pfizer lnr 235 East 42nd Street New York, NY 10007-5755 March 25. 2008 Steven Reynolds Director U.S. Nuclear Regulatory Coinmission Division of Nuclear Material Safet) 2443 Warrenville Road STE 2 IO Lisle. IL 60532-4352 -Re: Financial Assurance Demonstration for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the chief financial officer of Pfizer Inc., 235 East 42'ld Street, New York. New York 10017, a corporation. This letter is in support of this firm's use of the parent company guarantee financial test to demonstrate financial assurance, as specified in IO CFR Part 30. -

(R&D) Tax Credit
Report toThe the Pennsylvania Pennsylvania Department of General Revenue Assembly Bureau of Research on the Research and Development (R&D) Tax Credit The Pennsylvania Department of Revenue Bureau of Research March 15, 2012 Pennsylvania Research and Development Tax Credit Page 1 of 14 The Pennsylvania R&D Tax Credit Statute On May 7, 1997, Act 7 of 1997 created the Pennsylvania research and development (R&D) tax credit. The R&D tax credit provision became Article XVII-B of the Tax Reform Code of 1971 (TRC). The intent of the R&D tax credit was to encourage taxpayers to increase R&D expenditures within the Commonwealth in order to enhance economic growth. The terms and concepts used in the calculation of the Commonwealth’s R&D tax credit are based on the federal government’s R&D tax credit definitions for qualified research expense.1 For R&D tax credits awarded between December 1997 and December 2003, Act 7 of 1997 authorized the Department of Revenue (Department) to approve up to $15 million in total tax credits per fiscal year. Additionally, $3 million of the $15 million was set aside for “small” businesses, where a “small business” is defined as a “for-profit corporation, limited liability company, partnership or proprietorship with net book value of assets totaling…less than five million dollars ($5,000,000).” Over the years, several changes have been made to the R&D tax credit statute. Table 1 lists all of the acts that have changed the R&D tax credit statute, along with the applicable award years, the overall tax credit cap and the “small” business set aside. -

Research and Development at the University of Maryland Baltimore
RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT ANNUAL REPORT TABLE OF CONTENTS Overview 1 Extramural Funding at UMB 1 Office of Research and Development Outcomes 2 Success by School 6 Areas of Strength 13 Technology Commercialization 14 New Initiatives 19 Appendixes 20 David J. Ramsay, DM, DPhil President James L. Hughes Vice President, Research & Development www.ord.umaryland.edu RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE ANNUAL REPORT FY 02 RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT OVERVIEW The University of Maryland Baltimore reached a milestone number in extramural funding in FY 02: $305.3 million. The research conducted on campus and the hundreds of projects funded from federal, state and local governments and the private sector demonstrate UMB’s ever-increasing contribution to the life sciences and health care research fields. As an economic engine for Maryland, UMB provides access to millions of dollars of research to the business community. The commercial potential for much of this work is being actively patented and licensed. Summary of Success Principal Investigators with Awards: 617 Principal Investigators with $1 million Plus in Awards: 44 Funding Applications: 2,274 Funding Awards: 1,673 Total Dollar Amount of Extramural Funding: $305.3 Million Total Dollar Amount of Extramural Funding through ORD: $250 Million % of Total Extramural Funding: 82% Volume of Federal Awards: $142.7 Million NIH Funding Amount: $127.2 Million % of Indirect Costs: 22.8% EXTRAMURAL FUNDING AT UMB Support for extramural funding at the UMB campus has tripled in the last eight years. -

2014.10.22-110Cv00528-Pfizer-Etal
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC., PHARMACIA & UPJOHN ) COMPANY, PHARMACIA & UP JOHN ) COMPANY LLC, SUGEN, INC., C.P .. ) PHARMACEUTICALS INTERNATIONAL ) C.V., PFIZER PHARMACEUTICALS LLC, ) C.A. No. 10-528-GMS and PF PRISM C.V., ) ) Plaintiffs, ) ) v. ) ) MYLAN PHARMACEUTICALS INC., ) ) Defendant. ) _______________________________ ) MEMORANDUM I. INTRODUCTION In this patent infringement action, plaintiffs Pfizer Inc., Pharmacia & Upjohn Company, Pharmacia & Upjohn Company LLC, Sugen, Inc., C.P. Pharmaceuticals International C.V., Pfizer Pharmaceuticals LLC, and PF Prism C.V. (collectively, "Pfizer") allege that pharmaceutical products proposed by defendant Mylan Pharmaceuticals Inc. ("Mylan") infringe the asserted claims of the patents-in-suit. (D.I. 1.) The court held a four-day bench trial in this matter on November 26 through November 29, 2012. (D.I. 148-151.) Presently before the court are the parties' post-trial proposed findings of fact and conclusions of law concerning the validity of the patents-in-suit, specifically whether the asserted claims are invalid as obvious under 35 U.S.C. § 103. (D.I. 152, 153.) Pursuant to Federal Rule of Civil Procedure 52( a), and after having considered the entire record in this case and the applicable law, the court concludes that: (1) all asserted claims ofthe patents-in-suit are not invalid due to obviousness; and (2) Pfizer's Rule 52(c) motion is granted, and Mylan's Rule 52( c) motion is denied. These findings of fact and conclusions oflaw are set forth in further detail below. II. FINDINGS OF FACT 1 A. The Parties 1. Plaintiff Pfizer Inc. -

Optimizing Binding Kinetics in Medicinal Chemistry: Facts Or Fantasy?
Optimizing Binding Kinetics in Medicinal Chemistry: facts or fantasy? ‡ -Gon /RT kon e -G‡ /RT ‡ off ΔG on koff e -ΔGd/RT Kd e koff /kon P + L ‡ ΔG off ΔGd PL Gerhard Müller Ex-Head of Med Chem Mercachem, Nijmegen, NL Binding coordinate 11 The topic is hot, complex, and I am just an end-user & controversial “You can’t optimize koff, and you don’t need to optimize koff, you simply need to optimize Kd ! ” Head Med Chem, (top-5 Pharma), West Coast, US, Jan 2016 Optimizing Binding Kinetics in Medicinal Chemistry: facts or fantasy? ‡ -Gon /RT kon e -G‡ /RT ‡ off ΔG on koff e -ΔGd/RT Kd e koff /kon P + L ‡ ΔG off ΔGd PL Gerhard Müller Chief Scientific Officer Gotham Therapeutics, New YorkBinding coordinate 2 confidential Streetlight effect in medicinal chemistry Top-heavy distributions, rich-get-richer mechanisms 5% / 75% J. Med. Chem., 54 ,6405–6416 (2011) Vertex Pharmaceuticals, US J. Org. Chem. 73, 4443-4451 (2008) MW clogP shape clogP flatness Fsp3 flatland J. Med. Chem., 58, 2390−2405 (2015) para meta ortho J. Med. Chem., 59, 4443–4458 (2016) 3 Cheminformatics Analysis – Kinase Family Ligand and target promiscuity (ChEMBL21) Hu, Kunimoto, Bajorath Chemical Biology & Drug Design, 89, 834-845 (2017) quantitative kinome coverage 270 kinases with high-confidence data Top-10 kinases (45% of human kinome still unexplored) VEGFR2 TK 2239 erbB1 TK 1814 22.537 distinct IC50 values p38a CMGC 1509 9191 distinct scaffolds HGFR TK 1411 31.873 kinase-inhibitor combinations PI3a lipid 844 erbB-2 TK 768 GSK3b CMGC 743 SRC TK 709 Chk1 CAMK 693 -

SU14813: a Novel Multiple Receptor Tyrosine Kinase Inhibitor with Potent Antiangiogenic and Antitumor Activity
1774 SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity Shem Patyna,1 A.Douglas Laird, 3 Dirk B.Mendel, 5 KIT, and FLT3. In cellular assays, SU14813inhibited ligand- Anne-Marie O’Farrell,2 Chris Liang,6 dependent and ligand-independent proliferation, migration, Huiping Guan,8 Tomas Vojkovsky,6 Stefan Vasile,7 and survival of endothelial cells and/or tumor cells express- B Xueyan Wang,9 Jeffrey Chen,1 Maren Grazzini,1 ing these targets. SU14813inhibited VEGFR-2, PDGFR- , Cheng Y.Yang, 10 Joshua O˝.Haznedar, 5 and FLT3phosphorylation in xenograft tumors in a dose- 4 1 and time-dependent fashion. The plasma concentration Juthamas Sukbuntherng, Wei-Zhu Zhong, in vivo 2 1 required for target inhibition was estimated to be Julie M.Cherrington, and Dana Hu-Lowe 100 to 200 ng/mL. Used as monotherapy, SU14813 1Pfizer Global Research and Development; 2Phenomix Corp., exhibited broad and potent antitumor activity resulting in San Diego, California; 3Exelixis, Inc.; 4Celera Genomics, Inc., regression, growth arrest, or substantially reduced South San Francisco, California; 5Chiron Corp., Emeryville, growth of various established xenografts derived from 6 7 California; The Scripps Research Institute; The Burnham human or rat tumor cell lines. Treatment in combination Institute, La Jolla, California; 8AstraZeneca PLC, Waltham, Massachusetts; 9Metabolex, Inc., Hayward, California; and with docetaxel significantly enhanced both the inhibition 10Gilead Sciences, Inc., Foster City, California of primary tumor growth and the survival of the tumor- bearing mice compared with administration of either agent alone. In summary, SU14813inhibited target RTK Abstract activity in vivo in association with reduction in angio- Receptor tyrosine kinases (RTK), such as vascular endo- genesis, target RTK-mediated proliferation, and survival thelial growth factor receptor (VEGFR), platelet-derived of tumor cells, leading to broad and potent antitumor growth factor receptor (PDGFR), stem cell factor receptor efficacy. -

February 19, 2014
CANCER PREVENTION AND RESEARCH INSTITUTE OF TEXAS Oversight Committee Meeting February 19, 2014 CANCER PREVENTION AND RESEARCH INSTITUTE OF TEXAS Summary Overview of the February 19, 2014, Oversight Committee Meeting Please find enclosed the meeting packet for the next meeting of the CPRIT Oversight Committee to be held on Wednesday, February 19, 2014, at 10:00 AM. This summary overview of major agenda items provides background on key issues for Committee consideration. CEO Report Wayne Roberts will present the CEO’s report and address issues assigned by the Oversight Committee at the January 24th meeting including reconstituting the University Advisory Committee (UAC) and proposed dashboard metrics for the agency. Chief Scientific Officer Program Portfolio Presentation and Grant Award Recommendations Dr. Margaret Kripke will present the Program Integration Committee’s recommendations for scientific research awards. The research continuation grant recommendations are the first grant applications to be considered under the “new” review process set out by SB 149. SB 149 changed the way that grant recommendations are formally approved. A vote by two-thirds of the Oversight Committee that are present and voting (i.e. not recused because of a conflict of interest) is required to approve each funding recommendation. If two-thirds of the Oversight Committee does not vote to approve an award recommendation, then a statement explaining the reason for not following the PIC’s recommendation must be included in the meeting minutes. Product Development Officer Program Portfolio Presentation and Grant Award Recommendations Kristen Doyle, acting Product Development Officer, and Dr. Jack Geltosky, CPRIT’s Product Development Review Council Chair, will discuss CPRIT’s product development portfolio and present the Chief Executive Officer’s recommendations for product development grant awards. -

Documenting the Biotechnology Industry in the San Francisco Bay Area
Documenting the Biotechnology Industry In the San Francisco Bay Area Robin L. Chandler Head, Archives and Special Collections UCSF Library and Center for Knowledge Management 1997 1 Table of Contents Project Goals……………………………………………………………………….p. 3 Participants Interviewed………………………………………………………….p. 4 I. Documenting Biotechnology in the San Francisco Bay Area……………..p. 5 The Emergence of An Industry Developments at the University of California since the mid-1970s Developments in Biotech Companies since mid-1970s Collaborations between Universities and Biotech Companies University Training Programs Preparing Students for Careers in the Biotechnology Industry II. Appraisal Guidelines for Records Generated by Scientists in the University and the Biotechnology Industry………………………. p. 33 Why Preserve the Records of Biotechnology? Research Records to Preserve Records Management at the University of California Records Keeping at Biotech Companies III. Collecting and Preserving Records in Biotechnology…………………….p. 48 Potential Users of Biotechnology Archives Approaches to Documenting the Field of Biotechnology Project Recommendations 2 Project Goals The University of California, San Francisco (UCSF) Library & Center for Knowledge Management and the Bancroft Library at the University of California, Berkeley (UCB) are collaborating in a year-long project beginning in December 1996 to document the impact of biotechnology in the Bay Area. The collaborative effort is focused upon the development of an archival collecting model for the field of biotechnology to acquire original papers, manuscripts and records from selected individuals, organizations and corporations as well as coordinating with the effort to capture oral history interviews with many biotechnology pioneers. This project combines the strengths of the existing UCSF Biotechnology Archives and the UCB Program in the History of the Biological Sciences and Biotechnology and will contribute to an overall picture of the growth and impact of biotechnology in the Bay Area. -

Drug Repositioning: Extracting Added Value from Prior R&D Investments
Drug Repositioning: Extracting Added Value from Prior R&D Investments Hermann A.M. Mucke, Ph.D. InsightPharmaReports.com Drug Repositioning: Extracting Added Value from Prior R&D Investments Hermann A.M. Mucke, Ph.D. Published in July 2010 by Cambridge Healthtech Institute • www.InsightPharmaReports.com • Reproduction prohibited i Insight Pharma Reports is a division of Cambridge Healthtech Institute, a world leader in life science information and analysis through conferences, research reports, and targeted publications. Insight Pharma Reports focus on pharmaceutical R&D—the technologies, the companies, the markets, and the strategic business impacts. They regularly feature interviews with key opinion leaders; surveys of the activities, views, and plans of individuals in industry and nonprofit research; and substantive assessments of technologies and markets. Managers at the top 50 pharma companies, the top 100 biopharma companies, and the top 50 vendors of tools and services rely on Insight Pharma Reports as a trusted source of balanced and timely information. Related Report Data Mining in Drug Development and Translational Medicine by Hermann A.M. Mucke, Ph.D. General Manager: Alfred R. Doig, Jr. 781-972-1348, [email protected] Editorial Operations Director: Laurie Sullivan 781-972-1353, [email protected] Design Director: Tom Norton 781-972-5440, [email protected] Production Director: Ann Handy 781-972-5493, [email protected] Marketing Manager: James Prudhomme 781-972-5486, [email protected] Customer Service: Rose LaRaia 781-972-5444, [email protected] Corporate Subscriptions: David Cunningham 781-972-5472, [email protected] Global Report Sales: Jack Valeri 781-972-1355, [email protected] Insight Pharma Reports, 250 First Ave., Suite 300, Needham, MA 02494 www.InsightPharmaReports.com ii • www.InsightPharmaReports.com • Reproduction prohibited Drug Repositioning: Extracting Added Value from Prior R&D Investments Hermann A.M. -

Pfizer, Inc., Letter of Support for Parent
Ptizer Inc 235 East 42nd Street New York, NY 1OOl7-5755 March 25,2008 Steven Reynolds Director U.S.Nuclear Regulatory Commission Division of Nuclear Material Safety 2443 Warrenville Road, STE 2 10 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia & Upjohn Co. (Kalamazoo, MI) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42ndStreet in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. ofthe ~oard Chief Executive Officer RECEIVED MAR 2 8 2008 .' . Pfizer Inr 235 East 42nd Street New lork. NY 10007-5755 March 25, 2008 Steven Reynolds Director U .S . N uc I ear Reg u la t ory C om m i ss i on Division of Nuclear Material Safet) 2443 Warrenville Road, STE 2 IO Lisle, IL 60532-4352 -Re: Financial Assurance for Pharmacia & Upjohn Company LLC (Kalainazoo, MI) Dear Mr. Reynolds: 1 am the chief financial officer of Pfizer Inc.. 235 East 42'IdStreet, New York.