UR-Coeus Sponsor List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2009: Turning the Corner
data page 2009: Turning the corner Walter Yang Despite the shaky start to 2009, the biotech sector regained its financial Although initial public offerings showed signs of resuscitation (at least footing. Biotech indices were up, as were offerings and partnership mon- 13 more companies are now in the queue), follow-on financings came in ies. Excluding collaborations, the sector raised a total of $24.3 billion. above $6 billion—the second-best year over the past decade. Stock market performance Global biotech industry financing Although biotech indices were up ~16% last year, they underperformed The boost in partnership promises to US biotechs and follow-on financings other major indices. pushed industry funding to $61.3 billion, up 82% from 2008. 1,500 Swiss Market S&P 500 2009 36.9 10.0 5.1 2.2 6.0 0.9 1,400 NASDAQ Biotech Dow Jones 2008 20.0 3.2 5.3 3.1 1.9 0.1 1,300 NASDAQ BioCentury 100 2007 22.4 11.7 6.8 4.7 4.4 3.0 1,200 Partnering 2006 19.8 11.9 5.6 4.7 5.6 2.0 1,100 Year Debt and other Index 17.3 6.1 5.4 2.7 4.8 1.9 1,000 2005 Venture capital PIPEs 900 2004 10.9 8.8 5.3 2.9 3.3 2.6 Follow-ons 800 2003 8.9 9.1 4.0 2.2 3.9 0.5 IPOs 700 010203040506070 Amount raised ($ billions) 1/09 2/09 3/09 4/09 5/09 6/09 7/09 8/09 9/09 12/08 Month ending 10/09 11/09 12/09 Partnership figures are for deals involving a US company. -
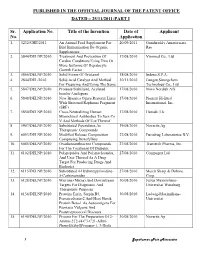
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should Be Applied to the Sellers of Pharmaceutical Products? Richard C
Kentucky Law Journal Volume 78 | Issue 4 Article 3 1990 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? Richard C. Ausness University of Kentucky Follow this and additional works at: https://uknowledge.uky.edu/klj Part of the Consumer Protection Law Commons, and the Food and Drug Law Commons Right click to open a feedback form in a new tab to let us know how this document benefits you. Recommended Citation Ausness, Richard C. (1990) "Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products?," Kentucky Law Journal: Vol. 78 : Iss. 4 , Article 3. Available at: https://uknowledge.uky.edu/klj/vol78/iss4/3 This Article is brought to you for free and open access by the Law Journals at UKnowledge. It has been accepted for inclusion in Kentucky Law Journal by an authorized editor of UKnowledge. For more information, please contact [email protected]. Kentucky Law Journal Volume 78 1989-90 Number 4 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? BY RICHARD C. AusNEss* Table of Contents INTRODUCTION ....................................... 706 I. OVERVIEW ................................ 709 A. Strict Products Liability .............................. 709 B. Comment K and "Unavoidably Unsafe" Products ................................................... 712 II. COM[ENT K's LIABILITY REGm .......................... 719 A. Risks Associated with the Production Process. 720 B. Risks Arising from the Inherent Nature of a Product .................................................... 723 C. Risks Created by Conscious Design Choices .... 726 D. Scientifically Unknowable Risks ................... 731 E. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation
www.sciencemag.org/cgi/content/full/327/5963/348/DC1 Supporting Online Material for Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation Jason Rihel,* David A. Prober, Anthony Arvanites, Kelvin Lam, Steven Zimmerman, Sumin Jang, Stephen J. Haggarty, David Kokel, Lee L. Rubin, Randall T. Peterson, Alexander F. Schier* *To whom correspondence should be addressed. E-mail: [email protected] (A.F.S.); [email protected] (J.R.) Published 15 January 2010, Science 327, 348 (2010) DOI: 10.1126/science.1183090 This PDF file includes: Materials and Methods SOM Text Figs. S1 to S18 Table S1 References Supporting Online Material Table of Contents Materials and Methods, pages 2-4 Supplemental Text 1-7, pages 5-10 Text 1. Psychotropic Drug Discovery, page 5 Text 2. Dose, pages 5-6 Text 3. Therapeutic Classes of Drugs Induce Correlated Behaviors, page 6 Text 4. Polypharmacology, pages 6-7 Text 5. Pharmacological Conservation, pages 7-9 Text 6. Non-overlapping Regulation of Rest/Wake States, page 9 Text 7. High Throughput Behavioral Screening in Practice, page 10 Supplemental Figure Legends, pages 11-14 Figure S1. Expanded hierarchical clustering analysis, pages 15-18 Figure S2. Hierarchical and k-means clustering yield similar cluster architectures, page 19 Figure S3. Expanded k-means clustergram, pages 20-23 Figure S4. Behavioral fingerprints are stable across a range of doses, page 24 Figure S5. Compounds that share biological targets have highly correlated behavioral fingerprints, page 25 Figure S6. Examples of compounds that share biological targets and/or structural similarity that give similar behavioral profiles, page 26 Figure S7. -

Fibrolase: Trials and Tribulations
Toxins 2010, 2, 793-808; doi:10.3390/toxins2040793 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Review Fibrolase: Trials and Tribulations Francis S. Markland 1,2,* and Steve Swenson 1,2 1 Department of Biochemistry and Molecular Biology, Cancer Research Laboratory, Keck School of Medicine, University of Southern California, 1303 N. Mission Rd., Los Angeles, CA 90033, USA 2 USC/Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-(323) 224-7981; Fax: +1-(323) 224-7679. Received: 11 March 2010; in revised form: 31 March 2010 / Accepted: 19 April 2010 / Published: 20 April 2010 Abstract: Fibrolase is the fibrinolytic enzyme isolated from Agkistrodon contortrix contortrix (southern copperhead snake) venom. The enzyme was purified by a three-step HPLC procedure and was shown to be homogeneous by standard criteria including reverse phase HPLC, molecular sieve chromatography and SDS-PAGE. The purified enzyme is a zinc metalloproteinase containing one mole of zinc. It is composed of 203 amino acids with a blocked amino-terminus due to cyclization of the terminal Gln residue. Fibrolase shares a significant degree of homology with enzymes of the reprolysin sub-family of metalloproteinases including an active site homology of close to 100%; it is rapidly inhibited by chelating agents such as EDTA, and by alpha2-macroglobulin (). The enzyme is a direct-acting thrombolytic agent and does not rely on plasminogen for clot dissolution. Fibrolase rapidly cleaves the A()-chain of fibrinogen and the B()-chain at a slower rate; it has no activity on the -chain. -

Guidelines on CVD During Pregnancy (TF17) - Task Force Members and Additional Contributors
Guidelines on CVD during Pregnancy (TF17) - Task Force Members and Additional Contributors Expert Relationship Type of relationship with industry Financial declaration with Industry Blomstrom-Lundqvist Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. Carina - Medtronic : Pacemaker B - Payment to your Institution: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Atricure : AF ablation Borghi Claudio Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Menarini International : ACE-inhibitors - Boheringer Ingelheim : Antihypertensive drugs - Recordati International : Antihypertensive drugs - Novartis : Antihypertensive, Heart Failure D - Research funding (departmental or institutional). - Barilla Food Company : Lactotripeptides Cifkova Renata Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Boehringer-Ingelheim : cardiovascular - Daiichi Sankyo : cardiovascular - MSD-SP, Boehringer Ingelheim, Bayer : cardiovascular B - Payment to your Institution: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Daiichi Sankyo : cardiovascular - Novartis : cardiovascular D - Research funding (departmental or institutional). - Krka Czech Republic : cardiovascular - Servier Czech Republic : cardiovascular Ferreira J Rafael No -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

Pfizer Inr 235 East 42Nd Street New York
Pfizer Inr 235 East 42nd Street New York. !W 10007-5755 March 25,2008 Steven Reynolds Director U.S. Nuclear Regulatory Commission Division of Nuclear Materials Safety 2443 Warrenville Road, STE 210 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42"d Street in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. panof the Board C ief Executive Officer Pfizer lnr 235 East 42nd Street New York, NY 10007-5755 March 25. 2008 Steven Reynolds Director U.S. Nuclear Regulatory Coinmission Division of Nuclear Material Safet) 2443 Warrenville Road STE 2 IO Lisle. IL 60532-4352 -Re: Financial Assurance Demonstration for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the chief financial officer of Pfizer Inc., 235 East 42'ld Street, New York. New York 10017, a corporation. This letter is in support of this firm's use of the parent company guarantee financial test to demonstrate financial assurance, as specified in IO CFR Part 30. -

Other Statutory Disclosures Continued
Other statutory disclosures continued Strategic reportGroup companies Governance & remuneration Financial statements In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the country of incorporation and effective percentage of equity owned, as at 31 December 2015 are disclosed below. Unless otherwise stated the share capital disclosed comprises ordinary shares which are indirectly held by GlaxoSmithKline plc. All subsidiary companies are resident for tax purposes in their country of incorporation unless otherwise stated. Country of Effective % % Held by Name incorporation Ownership Security Class of Share Wholly owned subsidiaries 1506369 Alberta ULC Canada 100 Common 100 Action Potential Venture Capital Limited England & Wales 100 Ordinary 100 Adechsa GmbH Switzerland 100 Ordinary 100 Affymax Research Institute United States 100 Common 100 Alenfarma – Especialidades Farmaceuticas, Limitada (iv) Portugal 100 Ordinary Quota 100 Allen & Hanburys Limited (iv) England & Wales 100 Ordinary 100 Allen & Hanburys Pharmaceutical Nigeria Limited Nigeria 100 Ordinary 100 Allen Farmaceutica, S.A. Spain 100 Ordinary 100 Allen Pharmazeutika Gesellschaft m.b.H. Austria 100 Ordinary 100 Aners S.A (iv) Argentina 100 Non-endorsable Nominative Ordinary 100 Barrier Therapeutics, Inc. United States 100 Common 100 Beecham Group p l c England & Wales 100 20p Shares 'A'; 5p Shares B 100 Beecham Pharmaceuticals (Pte) Limited Singapore 100 Ordinary 100 Beecham Pharmaceuticals S.A (iv) (vi) Ecuador 100 Nominative 100 Beecham Portuguesa-Produtos Farmaceuticos e Quimicos, Lda Portugal 100 Ordinary Quota 100 Beecham S.A. (iv) Belgium 100 Ordinary 100 Biddle Sawyer Limited India 100 Equity 100 Biovesta Ilaçlari Ltd. Sti. Turkey 100 Nominative 100 Burroughs Wellcome & Co (Australia) Pty Limited (iv) (vi) Australia 100 Ordinary 100 Burroughs Wellcome & Co (Bangladesh) Limited Bangladesh 100 Ordinary 100 Burroughs Wellcome International Limited England & Wales 100 Ordinary 100 Caribbean Chemical Company, Ltd. -

Research and Development at the University of Maryland Baltimore
RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT ANNUAL REPORT TABLE OF CONTENTS Overview 1 Extramural Funding at UMB 1 Office of Research and Development Outcomes 2 Success by School 6 Areas of Strength 13 Technology Commercialization 14 New Initiatives 19 Appendixes 20 David J. Ramsay, DM, DPhil President James L. Hughes Vice President, Research & Development www.ord.umaryland.edu RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE ANNUAL REPORT FY 02 RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT OVERVIEW The University of Maryland Baltimore reached a milestone number in extramural funding in FY 02: $305.3 million. The research conducted on campus and the hundreds of projects funded from federal, state and local governments and the private sector demonstrate UMB’s ever-increasing contribution to the life sciences and health care research fields. As an economic engine for Maryland, UMB provides access to millions of dollars of research to the business community. The commercial potential for much of this work is being actively patented and licensed. Summary of Success Principal Investigators with Awards: 617 Principal Investigators with $1 million Plus in Awards: 44 Funding Applications: 2,274 Funding Awards: 1,673 Total Dollar Amount of Extramural Funding: $305.3 Million Total Dollar Amount of Extramural Funding through ORD: $250 Million % of Total Extramural Funding: 82% Volume of Federal Awards: $142.7 Million NIH Funding Amount: $127.2 Million % of Indirect Costs: 22.8% EXTRAMURAL FUNDING AT UMB Support for extramural funding at the UMB campus has tripled in the last eight years.