05/01/02 Louisiana Medicaid Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should Be Applied to the Sellers of Pharmaceutical Products? Richard C
Kentucky Law Journal Volume 78 | Issue 4 Article 3 1990 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? Richard C. Ausness University of Kentucky Follow this and additional works at: https://uknowledge.uky.edu/klj Part of the Consumer Protection Law Commons, and the Food and Drug Law Commons Right click to open a feedback form in a new tab to let us know how this document benefits you. Recommended Citation Ausness, Richard C. (1990) "Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products?," Kentucky Law Journal: Vol. 78 : Iss. 4 , Article 3. Available at: https://uknowledge.uky.edu/klj/vol78/iss4/3 This Article is brought to you for free and open access by the Law Journals at UKnowledge. It has been accepted for inclusion in Kentucky Law Journal by an authorized editor of UKnowledge. For more information, please contact [email protected]. Kentucky Law Journal Volume 78 1989-90 Number 4 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? BY RICHARD C. AusNEss* Table of Contents INTRODUCTION ....................................... 706 I. OVERVIEW ................................ 709 A. Strict Products Liability .............................. 709 B. Comment K and "Unavoidably Unsafe" Products ................................................... 712 II. COM[ENT K's LIABILITY REGm .......................... 719 A. Risks Associated with the Production Process. 720 B. Risks Arising from the Inherent Nature of a Product .................................................... 723 C. Risks Created by Conscious Design Choices .... 726 D. Scientifically Unknowable Risks ................... 731 E. -

Health Industry Business Communications Council
Health Industry Business Communications Council Registered Labelers: Accredited Auto-ID Labeling Standards Argentina New MedTek Devices Pty Ltd Oxavita SRL Norseld Pty Ltd. Novadien Healthcare Pty Ltd The following companies Odontit S.A. (and/or their subsidiaries/ PAMPAMED S.R.L. Numedico Technologies Pty Ltd divisions) have applied PATEJIM SRL Opto Global Pty. Ltd. for a Labeler Identification Orthocell Limited Code (LIC) assignment with Austria Prolotus Technologies Pty Ltd HIBCC*. By doing so, they afreeze GmbH Red Milawa Pty Ltd dba Magic Mobility have demonstrated their AMI GmbH SDI Limited commitment to patient safety Bender Medsystems GmbH Signostics Ltd. and logistical efficiency for BHS Technologies GmbH Sirtex Medical Pty Ltd their customers, the industry Metasys Medizintechnik GmbH Smith & Nephew Surgical Pty. Ltd. and the public at large. PAA Laboratories GmbH Staminalift International Limited Safersonic Medizinprodukte Handels The Pipette Company Pty. Ltd. Any organization that is GmbH Thermo Electron Corporation interested in using the HIBC W & H Dentalwerk Burmoos GmbH Vush Pty Ltd uniform labeling system may apply for the assignment of VUSH STIMULATION Australia one or more LICs. William A Cook Australia Pty. Ltd. Adv. Surgical Design & Manufacture, Ltd. Last updated 9-21-2021 AirPhysio Pty Ltd Belgium Annalise-AI Pty Ltd 3M Europe Apollo Medical Imaging Technology Pty Advanced Medical Diagnostics SA/NV Ltd Analis SA/NV Benra Pty Ltd dba Gelflex Laboratories Baxter World Trade Bioclone Australia Pty. Ltd. Bio-Rad RSL Candelis, Inc. Bio-Rad Lab Inc Clinical Diag. Group DePuy Australia Pty. Ltd. Biosource Europe SA For more information, please dorsaVi Ltd Cilag NV contact the HIBCC office at: EC Certification Service GmbH Coris Bioconcept Fink Engineering Pty Ltd Fuji Hunt Photographic Chemicals NV 2525 E. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -
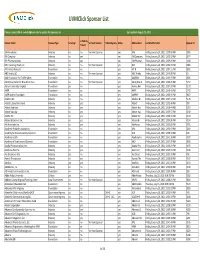
Sponsor List
UVMClick Sponsor List Please contact SPA at [email protected] to update the Sponsor List last updated August 24, 2021 Is Publicly Sponsor Name Sponsor Type Is Foreign Vermont Sponsor Federal Agency Active Abbreviation Last Modified Date Sponsor ID Traded 106 Associates Industry no no Vermont Sponsor yes 106 Friday, January 29, 2021 12:35:03 PM 3385 3M Company Industry no yes yes 3M Company Friday, January 29, 2021 12:32:32 PM 2877 3M Pharmaceuticals Industry no yes yes 3M Pharmac Friday, January 29, 2021 12:39:23 PM 4188 835 Hinesburg Road, LLC Industry no no Vermont Sponsor yes 835 Friday, January 29, 2021 12:32:33 PM 3386 A Territory Resource Foundation no no yes A.T.R. Friday, January 29, 2021 12:34:11 PM 3805 A&C Realty LLC. Industry no no Vermont Sponsor yes A&C Realty Friday, January 29, 2021 12:40:28 PM 60 AAA Foundation for Traffic Safety Foundation no no yes AAAFDN Friday, January 29, 2021 12:36:49 PM 3806 AALV/New Farms for New Americans Foundation no no Vermont Sponsor yes AALV/New FFriday, January 29, 2021 12:32:25 PM 5154 Aarhus University Hospital Foundation yes no yes Aarhus Uni Friday, January 29, 2021 12:34:23 PM 5172 AARP Foundation no no yes AARP Friday, January 29, 2021 12:40:40 PM 2742 AARP Andrus Foundation Foundation no no yes AARPAF Friday, January 29, 2021 12:36:35 PM 3807 Abalone Bio Industry no no yes Abalone Bi Friday, January 29, 2021 12:37:14 PM 5178 Abbott Laboratories Fund Industry no yes yes Abbott Friday, January 29, 2021 12:32:46 PM 309 Abbott Nutrition Industry no yes yes Abbott Nut Friday, January 29, 2021 12:38:44 PM 5219 Abbott Vascular Industry no yes yes Abbott Vas Friday, January 29, 2021 12:35:57 PM 4192 AbbVie Inc. -

Eric M. White, O.D., Inc
ERIC M. WHITE, O.D., INC . 5075 Ruffin Road Suite B, San Diego, CA 92123 (858) 278-4720 Toll Free (866) 458-2062 FAX (858) 278-3640 [email protected] www.drericwhite.com California License #8611T STATEMENT OF CURRICULUM VITAE EDUCATION June 1982 Bachelor of Arts, Physiological Psychology University of California, San Diego; San Diego, California. May 1984 Bachelor of Science, Visual Science Southern California College of Optometry; Fullerton, California. May 1986 Doctor of Optometry Southern California College of Optometry; Fullerton, California. PRIVATE PRACTICE 1986 - 1989 Partnership; D.M. Rasmussen, O.D., F.A.A.O., Mission Village Medical Center, 3303 Ruffin Road, San Diego, California. 1989-2001 Private Practice Mission Village Medical Center, 3303 Ruffin Road, San Diego, California. 2001-Present Private Practice 5075 Ruffin Road, Suite B, San Diego, California CLINICAL ROTATIONS 1985 Southern California College of Optometry; Fullerton, California . Provided comprehensive vision care including contact lenses, vision therapy, low vision, ocular photography, electrodiagnostic services, auto perimetry, and disease detection. 1985 San Bernardino Juvenile Hall; San Bernardino, California. Provided screening and treatment of vision and learning disabilities among juveniles. 1985 D.M. Rasmussen, O.D., F.A.A.O.; San Diego, California. Assisted doctor on investigational studies, comprehensive vision care, contact lenses, low vision, autoperimetry, and disease detection . INTERNSHIP 1986 United States Naval Submarine Base, San Diego, California. Provided comprehensive vision care including fitting and care for extended wear contact lenses. 1986 U.S. Naval Medical Corps. Marine Corps Recruit Depot; San Diego, California. Provided comprehensive vision care including disease detection. 1986 Balboa Regional Naval Hospital San Diego, California. -
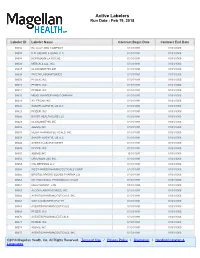
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC. -

Merrell Dow's Terfenadine Revisited)
The Unique Problem of Inventions Which Are Fully Enabled and Fully Described, But Not Fully Understood (Merrell Dow's Terfenadine Revisited) H. Samuel Frost of Bereskin & Parr 2007 Intellectual Property Journal October 2007 © Bereskin & Parr Bereskin & Parr 40 King Street West, 40th Floor, Toronto, Ontario, Canada M5H 3Y2 Phone: 416-364-7311 Fax: 416-361-1398 www.bereskinparr.com 1 Introduction ...........................................................................................................3 Inventions That are Not Fully Understood.........................................................3 Public Right to Use an Invention ...........................................................................4 Prior User Right.................................................................................................5 Novelty Requirement.........................................................................................6 Requirement for Fully Enabling Disclosure........................................................7 Patent Term.......................................................................................................8 Public Right to Use an Expired Invention ..........................................................8 The Dual Role of the Novelty Standard...............................................................10 Merrell Dow (Terfenadine) Case .........................................................................11 Germany..........................................................................................................11 -

Dr. Richard Rozek
Dear Ms. Overstreet: I am interested in being considered for the vacancy on the Glynn Brunswick Memorial Hospital Authority. Per the instructions with the vacancy announcement, I attached a copy of my vita to the letter. I am an economist with a specialty in health care economics. I have worked on competition, regulation, contract, and tax issues in health care during my career in academic, federal government, and private sector positions. I have written articles on health care issues and testified in major health care litigations. I have owned a home on Jekyll Island since 1993. Please let me know if you need additional information. Sincerely, Richard P. Rozek, Ph.D. RICHARD P. ROZEK, PH.D. CONTACT INFORMATION Redacted Jekyll Island, GA 31527 Phone: 912 Redacted Redacted gmail.com BACKGROUND Dr. Rozek received a B.A. degree in Mathematics with honors from the College of St. Thomas, a M.A. degree in Mathematics from the University of Minnesota, and M.A. and Ph.D. degrees in Economics from the University of Iowa. Dr. Rozek began his professional career as an Assistant Professor at the University of Pittsburgh where he taught courses in industrial organization, mathematical economics, and microeconomic theory. Dr. Rozek then worked for over six years in the Bureau of Economics at the Federal Trade Commission in a series of senior staff positions including Deputy Assistant Director for Antitrust. While at the FTC, Dr. Rozek evaluated antitrust and regulatory issues in electric and gas utilities, oil pipelines, soft drinks, for-profit and nonprofit hospitals, motion pictures, pharmaceuticals, and information industries. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

TAXOTERE (DOCETAXEL) MDL No
Case 2:16-cv-17144 Document 1 Filed 12/12/16 Page 1 of 101 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF LOUISIANA IN RE: TAXOTERE (DOCETAXEL) MDL No. 2740 PRODUCTS LIABILITY LITIGATION SECTION “N” (5) HON. KURT D. ENGLELHARDT BARBARA EARNEST Plaintiff, MAG. JUDGE NORTH vs. COMPLAINT & JURY DEMAND SANOFI S.A., Civil Action No. __________ AVENTIS PHARMA S.A., and SANOFI-AVENTIS U.S. LLC, separately, and doing business as WINTHROP U.S HOSPIRA WORLDWIDE, INC.; and SUN PHARMA GLOBAL INC.; and McKESSON CORPORATION d/b/a McKESSON PACKAGING; and SANDOZ INC.; and ACCORD HEALTHCARE INC.; and APOTEX, INC.; and PFIZER, INC.; and ACTAVIS PHARMA, INC.; and NORTHSTAR RX LLC; and EAGLE PHARMACEUTICALS, INC. Defendants. COMPLAINT AND JURY DEMAND Plaintiff, Barbara Earnest, by and through her attorneys, Bachus & Schanker, LLC, respectfully submits the following Complaint and Jury Demand against Defendants Sanofi S.A.; Aventis Pharma S.A.; and Sanofi-Aventis U.S. LLC, separately,; and doing business as Winthrop Case 2:16-cv-17144 Document 1 Filed 12/12/16 Page 2 of 101 U.S and Hospira Worldwide, Inc.; and Sun Pharma Global Inc.; and McKesson Corporation d/b/a McKesson Packaging; and Sandoz Inc.; and Accord Healthcare Inc..; and Apotex, Inc.; and Pfizer, Inc.; and Actavis Pharma, Inc.; and Northstar Rx LLC; and Eagle Pharmaceuticals, Inc., and alleges the following upon personal knowledge, information and belief, and investigation of counsel. NATURE OF THE ACTION 1. This action seeks to recover damages for injuries sustained by Plaintiff as the direct and proximate result of the wrongful conduct of Defendants Sanofi S.A., Aventis Pharma S.A., and Sanofi-Aventis U.S. -

United States District Court, S.D. Florida. MARION MERRELL DOW
Untitled Document 3/2/10 9:20 PM United States District Court, S.D. Florida. MARION MERRELL DOW INC., and Merrell Dow Pharmaceuticals, Inc, Plaintiffs. v. BAKER NORTON PHARMACEUTICALS, INC, Defendant. No. 94-1245-CIV-LENARD Nov. 12, 1996. Patentee brought action against generic drug manufacturer, alleging generic version of drug covered by expired patent infringed drug claim in unexpired patent. On motions for summary judgment, the District Court, Lenard, J., held that generic version of drug that was subject of expired patent did not literally infringe another, unexpired patent, which covered form of drug that would be metabolized in human liver after drug covered by expired patent was ingested. Judgment accordingly. 4,254,129. Not infringed. Gerald Sobel, Thomas L. Creel, and Joel Katcoff, Kaye, Scholer, Fierman, Hays & Handler, L.L.P., New York City, and John T. Kolinski, and Robert C. Fracasso, Shutts & Bowen, Miami, Florida, for Marion Merrell Dow, Inc. and Merrell Dow Pharmaceuticals, Inc. William L. Mentlik, Roy H. Wepner, and Arnold H. Krumholz, Lerner David Littenberg Krumholz & Mentlik, Westfield, New Jersey, and Alan H. Fein, Stearns Weaver Miller Weissler Alhadeff & Sitterson, PA, Miami, Florida, for Baker Norton Pharmaceuticals, Inc. ORDER ON MOTIONS FOR SUMMARY JUDGMENT LENARD, District Judge. Plaintiffs Marion Merrell Dow Inc. and Merrell Dow Pharmaceuticals, Inc. (collectively "MMD") filed this action for patent infringement against defendant Baker Norton Pharmaceuticals, Inc. ("Baker Norton"). Baker Norton counterclaimed for patent invalidity. Presently before the Court are (1) Baker Norton's Motion for Summary Judgment Based on Noninfringement of Patent (DE56), (2) MMD's Motion for Partial Summary Judgment on Noninfringement and for Dismissal of Baker Norton's Anticipation and Best Mode Defenses (DE183), (3) Baker Norton's Motion for Summary Judgment Based on Patent Invalidity (DE287), and (4) MMD's Motion for Preliminary Injunction (DE326).